| About Us | Contact Us | Calendar | Publish | RSS |
|---|
|
Features • latest news • best of news • syndication • commentary Feature Categories IMC Network:
Original Citieswww.indymedia.org africa: ambazonia canarias estrecho / madiaq kenya nigeria south africa canada: hamilton london, ontario maritimes montreal ontario ottawa quebec thunder bay vancouver victoria windsor winnipeg east asia: burma jakarta japan korea manila qc europe: abruzzo alacant andorra antwerpen armenia athens austria barcelona belarus belgium belgrade bristol brussels bulgaria calabria croatia cyprus emilia-romagna estrecho / madiaq euskal herria galiza germany grenoble hungary ireland istanbul italy la plana liege liguria lille linksunten lombardia london madrid malta marseille nantes napoli netherlands nice northern england norway oost-vlaanderen paris/Île-de-france patras piemonte poland portugal roma romania russia saint-petersburg scotland sverige switzerland thessaloniki torun toscana toulouse ukraine united kingdom valencia latin america: argentina bolivia chiapas chile chile sur cmi brasil colombia ecuador mexico peru puerto rico qollasuyu rosario santiago tijuana uruguay valparaiso venezuela venezuela oceania: adelaide aotearoa brisbane burma darwin jakarta manila melbourne perth qc sydney south asia: india mumbai united states: arizona arkansas asheville atlanta austin baltimore big muddy binghamton boston buffalo charlottesville chicago cleveland colorado columbus dc hawaii houston hudson mohawk kansas city la madison maine miami michigan milwaukee minneapolis/st. paul new hampshire new jersey new mexico new orleans north carolina north texas nyc oklahoma philadelphia pittsburgh portland richmond rochester rogue valley saint louis san diego san francisco san francisco bay area santa barbara santa cruz, ca sarasota seattle tampa bay tennessee urbana-champaign vermont western mass worcester west asia: armenia beirut israel palestine process: fbi/legal updates mailing lists process & imc docs tech volunteer projects: print radio satellite tv video regions: oceania united states topics: biotechSurviving Citieswww.indymedia.org africa: canada: quebec east asia: japan europe: athens barcelona belgium bristol brussels cyprus germany grenoble ireland istanbul lille linksunten nantes netherlands norway portugal united kingdom latin america: argentina cmi brasil rosario oceania: aotearoa united states: austin big muddy binghamton boston chicago columbus la michigan nyc portland rochester saint louis san diego san francisco bay area santa cruz, ca tennessee urbana-champaign worcester west asia: palestine process: fbi/legal updates process & imc docs projects: radio satellite tv |
printable version
- js reader version
- view hidden posts
- tags and related articles
View article without comments President George W. Bush: Will He Have to Step Aside for President Cheney? (Health)by C L Hallmark Saturday, Dec. 25, 2004 at 7:08 PMGeorge W. Bush apparently is wearing a medical device for "persons at risk of cardiac arrest." It is a LifeVest wearable defibrillator. He started using it sometime after his January 2002 fainting spell, which was attributed to choking. Based on photos showing him wearing the device, one can conclude the fainting was due to atrial fibrillation (AF), which his father also had.
PHOTOS Show George W. Bush Seriously Ill Physically
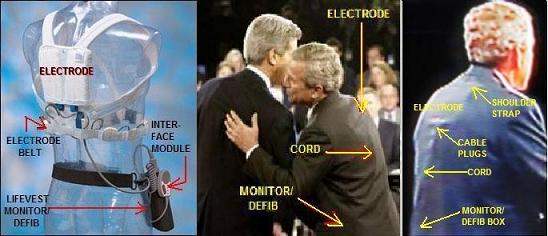
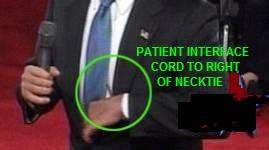
His father's AF was caused by Graves' hyperthyroidism, which Barbara Bush also has. Bush Jr likely has AF and, less likely, Graves', based on his family history and symptoms. The AF may have caused a stroke or TIA (mini-stroke), of which physicians watching the debates detected symptoms. Observers have noted psychological symptoms consistent with this and with Wernicke-Korsakoff disease. NOTE: If the photos have not uploaded to this site, see also: http://colombia.indymedia.org/news/2004/12/20372.php. [December 22, 2004] "The President remains in supurb physical condition," said Adam M. Robinson, Jr., commander of the National Naval Medical Center in Bethesda, MD, after the president's fourth annual physical at the center on December 11. "The doctors said that Mr. Bush had a 'low' to 'very low' risk of coronary artery disease, although they found evidence of minimal calcification of the coronary arteries themselves. As a preventative, they recommended that Mr. Bush take a daily aspirin and a statin, or cholesterol-lowering drug," reported the New York Times. It's interesting that his doctors advised him to take aspirin and statin. Aspirin is used to prevent clotting, which is a problem with AF (the condition he apparently wears the LifeVest for). Statins are the most potent cholesterol-lowering agents, lowering LDL (so-called "bad cholesterol) by 30-50 percent. They are less effective than fibrates in lowering triglycerides and raising HDL ("good cholesterol"). But Bush's doctors said he had a "low" to "very low" risk of heart disease, and his total cholesterol level was listed as 170 mg/dL, which is considered within the normal range. There is ongoing research into other areas where statins appear to have an effect, including dementia. Science Daily recently reported (11/17/04): "The cholesterol-lowering drug atorvastatin slowed down mental decline and improved depressive symptoms in people with Alzheimer’s disease" In contrast to the "fit for duty" report, the photos below show Mr. Bush at a presidential debate, with parts of a LifeVest wearable defibrillator clearly visible underneath his suit jacket. #file_1# The LifeVest (left) has an electrode belt with four sensing/ECG electrodes. These send signals to the heart monitor/defibrillator, typically worn like a holster. When the monitor detects a life-threatening heart arrhythmia, it sends a signal to the small, handheld patient-interface module (a little like a computer "mouse" but with just buttons and alarms). The module provides an audible alarm. The user, if able to do so, depresses two buttons on the module to hold off a shock from the defibrillator. If the user faints and is unable to press the buttons, the defibrillator sends an electrical pulse to the large shocking electrode on the patient's back and a smaller one on the chest. The pulse can be repeated until the heart starts pumping blood effectively, up to five pulses. In the center photo, showing Mr. Bush and Sen. Kerry at a debate, one can clearly see the shocking electrode between the shoulder blades as well as the electrical cord leading down to the monitor/defibrillator. (As for the small square patch near Mr. Bush's ear in this photo, I do not know. It is some kind of patch -- not a video artifact.) The photo on the right, from another debate, gives another view of the LifeVest components. Below we see the president adjusting his necktie, and as he does so, revealing a gray cord like that on the interface module ("mouse"), leading down to the monitor/defibrillator. The interface module possibly was located behind his tie. #file_2# This news could turn out to be the story of the year -- for 2005 -- if reporters ask the right questions to the right people. Some time between the fainting (pretzel) incident of January 2002 and the following summer, the president apparently began wearing the device. The photo of Mr. Bush at his ranch was taken on August 9, 2002. #file_3# THE FAINTING INCIDENT OF JANUARY 2002 On January 13, 2002, President Bush lost consciousness while sitting on a couch in the White House, watching a football game. His head hit the floor, resulting in an abrasion on his left cheekbone and a small bruise on his lower lip. The incident was blamed on a combination of (a) Mr. Bush not feeling well in previous days and (b) an improperly eaten pretzel. Their combined effect was to slow the president's heart. The description suggests a vasovagal attack: A pretzel lodged in his throat stimulated the vagus nerve to send a signal to his heart, slowing it down and reducing blood flow so much he fainted, according to White House physician Col. Richard J. Tubb, M.D. #file_4# Of course, even in this day of telemedicine, it is difficult and uncertain for anyone to diagnose a patient without having access to examination and test records. That doesn't stop physicians and psychologists from trying, especially on someone with President Bush's visibility. Obviously, considerable testing and examination have been done; but the American public does not always have the latest results, as they found out after other presidents, including Roosevelt and Kennedy, left office. Besides photographic evidence, there are observations and quotes from doctors, based on hours of Bush TV appearances, voluminous reports in the media, and the president's own words. ATRIAL FIBRILLATION (AF) In the January 2002 pretzel-choking episode, according to President Bush, the period of unconsciousness was brief -- a few seconds. When fainting begins and ends suddenly, the cause of fainting usually is not what his doctors reported (vasovagal syncope) but instead is an abnormal heart rhythm such as atrial fibrillation (AF). Chronic AF is consistent with Mr. Bush's requirement for constant monitoring and immediate access to defibrillation. Atrial fibrillation can lead to disastrous consequences if the patient is capable of sustaining a very rapid preexcited ventricular response with conduction over the accessory pathway. The rapid heart rate can produce syncope (fainting); or, more important, AF may cause ventricular fibrillation and sudden cardiac death. The LifeVest the president wears terminates ventricular fibrillation or ventricular tachycardia (overspeed) to prevent sudden cardiac death. This may be the reason the president wore the device during the debates, even though he risked exposing his vulnerability, especially if the device alarm sounded. According to the Framingham Offspring study of AF, a person whose parent had AF is 50 percent more likely to have it than the general population. President Bush's father had it during his presidency. While jogging at Camp David on a Saturday afternoon (May 4, 1991), Bush Sr developed shortness of breath, chest tightness, and a general feeling of fatigue. A White House physician discovered Bush had a rapid irregular heartbeat, ultimately diagnosed as atrial fibrillation caused by Graves' disease, a form of hyperthyroidism (overactive thyroid). Vagal-mediated AF is a well described phenomenon and often occurs in older athletes, which our current president, George W. Bush, is. As far as treatment, there are many different drug options. Blood thinners are often prescribed to reduce the risk of blood clots. President Bush's latest medical report (December 2004) included advice to take aspirin, which is a well known anti-clotting agent. Clotting can lead to strokes, discussed later. Although atrial fibrillation usually is controllable with treatment, it may become a lasting, chronic, condition. In the president's case, it apparently has, as his physicians evidently have decided to have him wear an external device that can continuously monitor his heart and shock it back into effective operation in case of an attack. Hyperthyroidism, hypertension, and other diseases can cause arrhythmias, as can recent heavy alcohol use (binge drinking). Some cases have no identifiable cause. The president says that he stopped drinking when he was 40, so binge drinking is not an issue. However, both of his parents have the Graves' disease form of hyperthyroidism and it is hereditary and must be considered. The earliest photo I have seen of President Bush wearing the LifeVest was the one taken in August 2002. He probably started wearing the LifeVest between the January 2002 pretzel incident and this. The most likely time would seem to be after his June 28, 2002 colonoscopy, for which he was put under anesthesia at Camp David, MD -- that is, some time in July 2002. At any rate, the president bears watching for symptoms of AF, which include: heart palpitations (sensation of rapid heartbeat), irregular pulse, shortness of breath (especially during physical activity or emotional stress), chest pain (angina), weakness and fatigue, dizziness and confusion, lightheadness, or confusion. If the condition remains untreated, serious complications may occur, including stroke, heart attack, and heart failure. EVIDENCE OF STROKE OR TIA The evidence of atrial fibrillation is strong, based on photographic evidence that Mr. Bush wears an anti-arrythmic device, his father's having it, a confirmed sudden-fainting spell, numerous falls, and observation-at-a-distance. The evidence of a stroke or TIA is less strong. It is symptomatic and based observation-at-a-distance -- TV appearances and news reports -- but it is there. A stroke or TIA (transient ischemic attack) is one possible consequence of AF itself. A TIA basically is a mini-stroke. Many observers, including physicians, have been concerned or even alarmed at the symptoms President Bush has evidenced in TV appearances. The photo below shows the drooping mouth noticed by Dr. W. Kendall Tongier, M.D. of Dallas, Texas. #file_5# After watching the third presidential debate, Dr. Tongier posted on the Dallas Morning News website about his concerns that the president may have had a stroke. Dr. Tongier has been an anesthesiologist for 15 years. His post said: "Having watched the first two debates from start to finish, I was looking forward to listening to a spirited debate between Bush and Kerry. Unfortunately, I barely heard a word that was said. Instead, I found myself staring at and concentrating on the president's drooping mouth." "As a physician and a professor, I tend to pick up on signs and symptoms of physical problems better than most other people. I am highly concerned with what I saw. The drooping left side of the President's face, his mouth and nasolabial fold (the crease in the face running from the nostril to the side of mouth) may be indicative of a recent stroke, TIA (transient ischemic attack) or, possibly botox injections. I sincerely hope this was nothing more than botox injections. The other options are truly scary given an upcoming election for president in three weeks." In a phone interview reported by Salon.com, Dr. Tongier stressed that he's not a neurologist, and no doctor can make a diagnosis from a 90-minute debate. But he did explain why he found Bush's face so distracting on TV: "It struck me across the face to the point where I wasn't really listening to the debate. It looked like the left side of his mouth was downturned. You know how he sneers at times. At first I thought that's what it was, but it didn't change when his face was at rest. It changed when he talked, but you'd expect that. It's the loss of muscle tone there that's really kind of concerning. And it was pretty much persistent throughout the entire debate." "It certainly could be something as benign as an overzealous botox injection, which causes the paralysis, which is essentially how botox works. A lot of people will get them around the nasolabial fold to decrease those lines. If it's botox, it can be a short-term reaction after an injection. It could last for 24 hours and be gone. But I'd like to see the Bush campaign at least give an explanation." Not only was there no explanation, but the president delayed his annual medical examination until after the election. Even now, as far as I know, the complete details, including tests made and the metrics of those tests, have not come out -- only a conclusion that he is in "supurb condition." If the speculation is true, the writers of the president's medical report should be held to account for the discrepancies. Atrial fibrillation is an irregular heartbeat in which the upper chamber of the heart quivers rapidly rather than beats. Even if it does not result in life-threatening ventricular fibrillation, the quivering motion is not forceful enough to send all the blood to the heart's lower chambers (ventricles), and the blood pools, thus allowing clots to develop. The clots can migrate elsewhere, including the neck or brain (in cerebral embolism), and they can cause strokes. A series of strokes can cause a progressive impairment of brain function known as "multi-infarct dementia." DEMENTIA The term "dementia" refers to a group of symptoms involving progressive impairment of all aspects of brain function. Disorders that cause dementia include conditions that impair the vascular (blood vessels) or neurologic (nerve) structures of the brain. Multi-infarct dementia results from the damage caused by strokes, and there is evidence that Mr. Bush has suffered multiple small strokes or TIAs. One other type of dementia is caused by Wernicke encephalopathy, which is bleeding and swelling of the brain due to multiple brain lesions, caused by lack of thiamine vitamin. The disease often is connected to alcoholism. Mr. Bush drank, often heavily, according many published reports, from about age 20 to age 40. He said himself one time that he couldn't remember a day he didn't have a drink. A minority of causes of dementia are treatable. Wernicke can be treated with thiamine. If untreated long enough, the disease progresses to Korsakoff's disease, which like most of the disorders associated with dementia are progressive, irreversible, degenerative conditions. The two major degenerative causes of dementia are (1) Alzheimer's disease, which is a progressive loss of nerve cells without a known cause or cure, and (2) vascular dementia, which is loss of brain function due to a series of small strokes. Vascular dementia may or may not play a role in the progression of Alzheimer's disease: the conditions often occur together and neither can be diagnosed definitively except until autopsy. In those with the genetic and environmental susceptibility to develop Alzheimer's disease, the concomitant presence of small infarcts (lacunar strokes) speeds up the onset of Alzheimer's disease to an earlier age than if it were to occur alone (i.e., without small infarcts). President Bush has been prescribed statins, which slow the progression of dementia, even though is over all cholesterol is within the normal range. Dementia may be diagnosed when there is impairment of two or more brain functions, including language, memory, visual-spatial perception, emotional behavior or personality, and cognitive skills (such as calculation, abstract thinking, or judgment). Dementia usually appears first as forgetfulness. Other symptoms may be apparent only on neurologic examination or cognitive testing. A well-respected psychoanalyst has written a psychological profile of the president, called "Bush on the Couch: Inside the Mind of the President" (ISBN: 0060736704). Justin A. Frank, M.D., is a clinical professor in the Department of Psychiatry at George Washington University Medical Center. Since 1980 he has been a teaching analyst at the Washington Psychoanalytic Institute. He is past president of the Greater Washington Chapter of Physicians for Social Responsibility. Dr. Frank lives and practices psychoanalysis in Washington, D.C. Among the problems Dr. Frank found in Mr. Bush are megalomania, characterized by a Manichaean worldview, delusions of persecution and omnipotence, and an "anal/sadistic" indifference to others’ pain. The Manichaean tradition is a defunct religion with a "good cop/bad cop" theology. The book follows Mr. Bush from childhood to now and analyzes the drinking problem, the bellicose rhetoric, the verbal flailings and misstatements of fact, the religiosity and exercise routines, the hints of dyslexia and hyperactivity, the youthful cruelty to animals and schoolmates Below are some of the problems associated with dementias, including vascular and alcoholic (Wernicke-Korsakoff syndrome). Some of the things in this list were observed by Dr. Frank in Mr. Bush. Other items exist further along in the progression of the disease. - progressive loss of memory - inability to concentrate - decrease in problem-solving skills and judgement capability - persistence in failed problem-solving modes, "staying the course" at all costs (perseveration) - confusion - hallucination, delusions - altered sensation or perception - impaired recognition (agnosia) of familiar objects or persons - altered sleep patterns - MOTOR SYSTEM IMPAIRMENT -----gait changes -----inappropriate movements -----other impairments of motor system - disorientation - inability to generalize, learn, think abstractly, or perform calculations - MEMORY DEFICIT ----- short term (can't remember new things) ----- long term (can't remember past) Persons with this may make up stories to cover up nothing but the memory lapse itself. (confabulation) - IMPAIRED LANGUAGE ABILITY ----- inability to comprehend speech ----- inability to read (alexia) ----- inability to write (agraphia) ----- inability to find words (aphasiia) ----- inability to repeat a phrase ----- persistent repetition of phrases or words (Much has been made of President Bush's use of the word "fabulous.") - PERSONALITY PROBLEMS ----- irritability ----- poor temper management ----- anxiety ----- depression ----- indecisiveness ----- self-centeredness ----- inflexibility ----- no observable mood (flat affect) ----- inappropriate mood or behavior ----- withdrawal from social interaction ----- inability to function in social or personal situations ----- lack of spontaneity Some of the above have been noted by Dr. Frank in the Bush book. Some have been noted by other MD's and by medical laymen, including members of the press. Evidence of motor-system impairment has been well chronicled by the media. Most of these incidents have come at times of particular stress. They include: January 2002: While watching a football game, Mr. Bush fainted and injured his face. This was a time of stress, 2 weeks before his post-9/11 State of the Union address. May 2003: Mr. Bush fell off a Sedgway gyroscopically balanced 2-wheel scooter. Another stress -- 2 weeks before a trip abroad to line up support for Iraq war. August 2003: Mr. Bush dropped his dog Barney as Mrs. Bush handed him the dog at the TSTC Airport in Waco, Texas, to the horror of onlooking schoolgirls. In little over a week he would give only his second (the first was to announce the start of the war on Iraq) address to the nation defending the yellowcake/Wilson/Plame matter, the WMD deadend, and the growing insurgency in Iraq -- arguably his most important national address to date. This was a time of stress. May 2004: Two days before one of very few and arguably the most important prime-time speech of his, in which he defended the Iraq war in the wake of Fallujah and Najaf unrest, Mr. Bush fell and injured his face again. His approval rating was 41 percent, the lowest of his presidency, at this time of stress. June 4, 2004: Mr. Bush appeared with a scatch on his right cheek. Had he fallen again? Additional symptoms that may be associated with this organic brain disease include swallowing problems. However, this and some of the above are also symptoms of hyperthyroidism. HYPERTHYROIDISM (OVERACTIVE THYROID) When one of a person's parents has hyperthyroidism, one cause of AF and the cause in his father's case, the chances are that 50 percent of their children will have it. Both of President Bush's parents have Graves' disease, increasing his chances of getting it even more. The president's father was diagnosed with Graves' disease, a form of hyperthyroidism in 1991, within 18 months of Barbara Bush being diagnosed with the same thing. The chances of this happening to two unrelated persons in such a short time is about 1 in 3 million. Because of the remarkable coincidence, the Secret Service tested the water in the White House, at Camp David, at the Vice President's residence, and at Walker's Point (Bush's home in Maine) for lithium and iodine, two substances "known to cause thyroid problems." The lead investigator was Dr. Kenneth Burman, then a colonel in the Army medical corps (the same man whom the media is quoting about the thyroid cancer of Chief Justice Rehnquist). Besides causing atrial fibrillation as it did in the case of Bush Sr and possibly Bush Jr, hyperthyroidism has the following symptoms: -- Feeling of fullness in the throat -- Enlargement of the thyroid, known as goiter -- CHOKING ON FOODS -- Irregularities in blood pressure and HEART RATE. -- FAINTING OR DIZZINESS -- SENSITIVITY TO LIGHT AND A CONTINUAL FEELING THAT THERE IS SOMETHING IN THE EYES (This could explain the president's rapid eye-blinking, noted in the presidential debates.) The onset of Graves' frequently is during times of intense stress. Bush Sr fell ill with Graves' and AF during the first Iraq war. The president's physicians should be looking carefully at his thyroid antibodies and conducting a complete thyroid bloodwork panel in view of his symptoms and family history. Of course, Mr. Bush should level with the American people on this. OTHER SYMPTOMS OF HYPERTHYROIDISM Enlarged thyroid gland Rapid heart beat (tachycardia) or heart palpitations ATRIAL FIBRILLATION (President Bush appears to have worn a LifeWest defibrillator since about July 2002.) Smooth, velvety skin Tremor of the fingertips Fatigue SWEATY PALMS -- This is related to heat intolerance. Weight loss Fine brittle hair RESTLESSNESS -- Some observers noted this during the debates. Depression Increased appetite Changes in sex drive MUSCLE WEAKNESS, ESPECIALLY IN THE UPPER ARMS AND THIGHS -- The president did drop his 15-pound dog during a handoff from his wife as mentioned above. Also, he has replaced a large part of his running regimen with mountain-bike riding, which, as noted, has resulted in one injurious accident. Shortened attention span. HEAT INTOLERANCE -- In the Bush-Kerry camps' agreement for the 2004 debates, one requirement was that the temperature be kept below 70 degrees. This requirement apparently came from the Kerry camp since Sen. Kerry supposedly sweats easily. However, this would also serve Mr. Bush's interest if he has hyperthyroidism. INCREASED SWEATING NERVOUSNESS AND IRRITABILITY -- This is one of the symptoms that also has a neurological etiology. Restless sleep or insomnia -- Mr. Bush does arise at 5 a.m., at least at his ranch. ERRATIC BEHAVIOR -- Besides hyperthyroidism, this obiously also is a symptoms of Wernick-Korsakoff syndrome (alcoholic syndrome) as well as various dementias including multi-infarct dementia. CONCLUSION The president apparently uses a wearable defibrillator, a device to stop heart arrhythmia, as seen in photographs. He has had a sudden fainting spell and other symptoms of atrial fibrillation (AF) and has a genetic tendency for it. Some observers have noted neurological and psychological irregularities and other evidence of stroke, which is a possible result of AF. Some of these irregularities could be caused by Wernicke-Korsakoff's, a disease of inveterate alcoholics, or some other organic brain disease. The president has an even stronger genetic predisposition for hyperthyroidism and some symptoms of it, including the heartbeat arrhythmia. Only his doctors know for sure.
Report this post as:
2by C L Hallmark Saturday, Dec. 25, 2004 at 7:08 PM
error
Report this post as:
3by C L Hallmark Saturday, Dec. 25, 2004 at 7:08 PM
error
Report this post as:
4by C L Hallmark Saturday, Dec. 25, 2004 at 7:08 PM
error
Report this post as:
5by C L Hallmark Saturday, Dec. 25, 2004 at 7:08 PM
error
Report this post as:
Inbreeding tellsby meat inspector Saturday, Dec. 25, 2004 at 10:33 PMI feel so sorry...
...that this defective is the best the Republicans can come up with as a semi talking, self propelled, meat puppet. Long live the chimp.
Report this post as:
other stroke symptomsby more rational Sunday, Dec. 26, 2004 at 3:38 AMAfter strokes, people get that droopy face, and have a few speech impairments. Speech may be slurred. Word choice may be affected, and the person may mistakenly choose a word that starts with the same letter or sound. For example, "hand me the cat" instead of "hand me the cup". They don't catch the error until after they've said the word.
Also, higher order thinking seems to be impaired, in the stroke victims I've met. They lose some sense of sentimentality, or subtlety of feeling. It can be refreshing, because they live "in the moment," and they really appreciate happy moments, but you can't count on them to really be sympathetic to others' pain. The lose interest in reading, if they can read at all. I've had two relatives die from strokes, and have met a few victims who are still living. I'll probably have my own in a few more decades. Oh well.
Report this post as:
deviceby Meyer London Tuesday, Dec. 28, 2004 at 1:41 PMI think the device is an artificial, battery-powered brain. Even if it is powered only by two AA batteries, they probably still deliver way more power than his original brain cells.
Report this post as:
yes, computers get more powerful each yearby more rational Tuesday, Dec. 28, 2004 at 3:48 PMIndeed, computers do get more powerful each year.
I saw these old electronic kits that made a simple battery powered version of the "mysterious 8-ball" executive decisionmaking tool. It was a lot faster than flipping that old ball upside down over and over.
Report this post as:
also aspartame (methanol, formaldehyde, formic acid)by Rich Murray Monday, Jan. 03, 2005 at 12:16 PMrmforall@comcast.net 505-501-2298 1943 Otowi Road, Santa Fe, New Mexico 87505 2005.01.04 Hello Clayton L. Hallmark,
[ Concerned that important information for the health of the President somehow reach responsible experts, I am sending this to researchers connected with National Naval Medical Center whose email addresses were in PubMed since May, 2003. ] [ http://la.indymedia.org/news/2004/12/120496.php President George W. Bush: Will He Have to Step Aside for President Cheney? (Health) George W. Bush apparently is wearing a medical device for "persons at risk of cardiac arrest." It is a LifeVest wearable defibrillator. He started using it sometime after his January 2002 fainting spell, which was attributed to choking. Based on photos showing him wearing the device, one can conclude the fainting was due to atrial fibrillation (AF), which his father also had. lifevest_duo_back_2.jpg, image/jpeg, 548x236 PHOTOS Show George W. Bush Seriously Ill Physically His father's AF was caused by Graves' hyperthyroidism, which Barbara Bush also has. Bush Jr likely has AF and, less likely, Graves', based on his family history and symptoms. The AF may have caused a stroke or TIA (mini-stroke), of which physicians watching the debates detected symptoms. Observers have noted psychological symptoms consistent with this and with Wernicke-Korsakoff disease. NOTE: If the photos have not uploaded to this site, see also: http://colombia.indymedia.org/news/2004/12/20372.php. ] Thanks for your detailed, competent article on the possibility that President Bush has Graves Disease, as do both his parents. I have published on the Net several reviews with much relevant information regarding the risk to him and other leaders from aspartame and other methanol (formaldehyde, formic acid) sources. http://groups.yahoo.com/group/aspartameNM/message/876 hyperthyroidism (Graves disease) in George and Barbara Bush, 1991-- aspartame toxicity? Roberts 1997: Murray 2002.10.09 rmforall In mutual service, Rich Murray, MA Room For All rmforall@comcast.net 1943 Otowi Road, Santa Fe, New Mexico 87505 USA 505-501-2298 http://groups.yahoo.com/group/aspartameNM/messages 137 members, 1,142 posts in a public searchable archive ***************************************************************** http://groups.yahoo.com/group/aspartameNM/message/1091 Justin Dumais, 25, Olympic diver, business graduate, pilot, healed of Graves disease (hyperthyroidism) by giving up aspartame: Roberts: Murray 2004.06.11 rmforall "The disease most often strikes middle-aged women. "A 25-year-old male elite athlete is about as far from the stereotypical Graves patient as you can get," he says. Now, he has his doctors baffled. He began taking medication in February but continued doing his own research. He found a nutritionist who suggested he cut aspartame, an artificial sweetener found in products such as diet soda, from his diet. In mid-March, he quit diet soda and his medication. Now, Dumais feels so much better, he questions whether he really has Graves' disease, which has no known cure." http://www.usatoday.com/sports/olympics/athens/swimming/2004-06-10-dumais_x.htm Posted 6/10/2004 10:03 PM Updated 6/11/2004 1:04 PM Disease diagnosis doesn't deter diver By Vicki Michaelis, USA TODAY ***************************************************************** http://groups.yahoo.com/group/aspartameNM/message/1092 Janet Starr Hull, who also had Graves disease in 1991, told Justin Dumais to quit aspartame: Murray 2004.06.12 rmforall From: "Dr. Janet Starr Hull" <jshull@sweetpoison.com> To: "Rich Murray" <rmforall@comcast.net> Subject: Re: Justin Dumais, 25, Olympic diver, business graduate, pilot,healed of Graves disease (hyperthyroidism) by giving up aspartame:Roberts: Murray 2004.06.11 rmforall Date: Saturday, June 12, 2004 6:37 PM Thanks so much for circulating Justin's wonderful case history. The nutritionist he found was myself, after he had read my book SWEET POISON. His case is identical to mine, and he followed my experience and my 10 Step Detox program exactly as I did, and his Grave's Disease disappeared within 60 days. We have been working closely for the past few months. Keep spreading this wonderful news. As Justin's story is yet another case identical to mine that proves neither of us ever had Grave's Disease -- merely aspartame poisoning. All the best, Janet Hull Dr. Janet Starr Hull, PhD, CN jshull@sweetpoison.com Hair Analysis Program: http://www.hairanalysisprogram.com http://www.sweetpoison.com/ http://www.janethull.com/about/index.php http://www.detoxprogram.net/ http://www.detoxprogram.net/about-dr-janet-hull.php ***************************************************************** Fully 11% of aspartame is methanol [ wood alcohol ] -- 1,120 mg aspartame in 2 L diet soda, almost six 12-oz cans, gives 123 mg methanol (wood alcohol) -- the same amount that produces hangover from red wine. The methanol is immediately released into the body after drinking -- as are the even larger levels of methanol locked up in complex pectin molecules inside many fruits and vegetables, released by degradation by bacteria in the colons of many people. Within hours, the body turns much of the methanol into formaldehyde, and then much of that into formic acid, both of which in time are partially eliminated as carbon dioxide and water. This long-term low-level chronic toxic exposure leads to typical patterns of increasingly severe complex symptoms, starting with headache, fatigue, joint pain, irritability, memory loss, rashes, and leading to vision and eye problems, and even seizures. In many cases there is addiction. Probably there are immune system disorders, with a hypersensitivity to these toxins and other chemicals. groups.yahoo.com/group/aspartameNM/message/1065 politicians and celebrities hooked on diet sodas (aspartame): Murray 2004.03.24 rmforall groups.yahoo.com/group/aspartameNM/message/1121 diet soda sweetener aspartame [ NutraSweet, Equal, Canderel, E951, Benevia ] toxicity research: Murray 2004.10.11 rmforall Rich Murray, MA Room For All rmforall@comcast.net 1943 Otowi Road, Santa Fe, New Mexico 87505 USA 505-501-2298 groups.yahoo.com/group/aspartameNM/messages 137 members, 1,125 posts in a public searchable archive Moderator [ BS physics and history MIT 1964, MA psychology Boston University Graduate School 1967, 1988 to present, full-time home hospice care giver in Santa Fe ] ************************************************************** http://groups.yahoo.com/group/aspartameNM/message/927 Donald Rumsfeld, 1977 head of Searle Corp., got aspartame FDA approval: Turner: Murray 2002.12.23 rmforall http://groups.yahoo.com/group/aspartameNM/message/1016 President Bush & formaldehyde (aspartame) toxicity: Ramazzini Foundation carcinogenicity results Dec 2002: Soffritti: Murray 2003.08.03 rmforall http://groups.yahoo.com/group/aspartameNM/message/874 re "dry drunk": Bisbort: danger to President Bush from aspartame toxicity: Murray: 2002.02.24 2002.09.29 rmforall http://www.willthomas.net/911/Bush/ Is Bush Nuts?: William Thomas willthomas.net 2004 http://groups.yahoo.com/group/aspartameNM/message/1101 John Edwards gives up Diet Coke: The Cult of Diet Coke, Eric Gillin: Murray 2004.07.12 rmforall http://groups.yahoo.com/group/aspartameNM/message/1102 John Edwards still drinks Diet Coke (aspartame): TIME Europe July 19 issue: Murray 2004.07.12 rmforall http://groups.yahoo.com/group/aspartameNM/message/876 hyperthyroidism (Graves disease) in George and Barbara Bush, 1991-- aspartame toxicity? Roberts 1997: Murray 2002.10.09 rmforall http://www.dorway.com/upipart1.txt http://groups.yahoo.com/group/aspartameNM/message/262 aspartame expose 96K Oct 1987 Part 1/3: Gregory Gordon, UPI reporter: Murray 2000.07.10 rmforall http://www.dorway.com/enclosur.html http://groups.yahoo.com/group/aspartameNM/message/53 aspartame history Part 1/4 1964-1976: Gold: Murray 1999.11.06 rmforall http://groups.yahoo.com/group/aspartameNM/message/928 revolving door, Monsanto, FDA, EPA: NGIN: Murray 2002.12.23 rmforall *************************************************************** I have been writing careful reviews of mostly mainstream medical research on aspartame toxicity for six years. In recent months I have become aware that evidence strongly shows that substantial methnol and thus formaldehyde and formic acid are released into humans from degradation of the pectins from fruits and vegetables by bacteria in the colon in many people. The biochemical dispositions of formaldehyde are actually largely unknown, according to the expert comprehensive review by Bouchard M et al, 2001. Research on hangovers, largely caused by the conversion of methanol impurity in alcohol drinks into formaldehyde after about eight hours, shows that a quarter to a half of those who get inebriated do not get hangovers. This shows very large individual variation in vulnerability to formaldehyde toxicity, so as a corollary, probably there will be many who are not markedly vulnerable to aspartame. I am guessing that evolution has given humans the ability to store the large amounts of formaldehyde generated from pectins and to use them to attack pathogens. So far, I have not found any strong research to support this hypothesis. I hope to write a useful summary in the next few weeks. There are many substances, such as folic acid, that protect against formaldehyde toxicity. http://groups.yahoo.com/group/aspartameNM/message/1143 antiseptic? antifungal? antiviral? methanol (formaldehyde, formic acid) disposition: Bouchard M et al, full plain text, 2001: substantial sources are degradation of fruit pectins, liquors, aspartame, smoke: Murray 2005.01.04 rmforall This long, complex review presents mainstream evidence for several ubiquitous, substantial sources of methanol and its inevitable chain of products, formaldehyde and formic acid, which I will initialize as "MCC", for Methanol Chain Compounds. Monte WC in his seminal summary review (1984) mentions that humans are uniquely vulnerable to the conversion of methanol into formaldehyde: "Fruit and vegetables contain pectin with variable methyl ester content. However, the human has no digestive enzymes for pectin (6, 25) particularly the pectin esterase required for its hydrolysis to methanol (26)." "Humans, due perhaps to the loss of two enzymes during evolution, are more sensitive to methanol than any laboratory animal; even the monkey is not generally accepted as a suitable animal model (42)." "The methyl ester bond of phenyalanine is the first to cleave due to its susceptibility to pancreatic enzymes (40). This is highly unusual; the methyl esters associated with pectin for instance are completely impervious to all human digestive enzymes (6)." "The greater toxicity of methanol to man is deeply rooted in the limited biochemical pathways available to humans for detoxification. The loss of uricase (EC 1.7.3.3.), formyl-tetrahydrofolate synthetase (EC 6.3.4.3.) (42) and other enzymes (18) during evolution sets man apart from all laboratory animals including the monkey (42)." "The importance of ethanol as an antidote to methanol toxicity in humans is very well established in the literature (46, 55). The timely administration of ethanol is still considered a vital part of methanol poisoning management (11, 12, 19, 20, 50). Ethanol slows the rate of methanol's conversion to formaldehyde and formate, allowing the body time to excrete methanol in the breath and urine. Inhibition is seen in vitro even when the concentration of ethyl alcohol was only 1/16th that of methanol (62). The inhibitory effect is a linear function of the log of the ethyl alcohol concentration, with a 72% inhibition rate at only a 0.01 molar concentration of ethanol (2, 46). Oxidation of methanol, like that of ethanol, proceeds independently of the blood concentration, but at a rate only one seventh (20) to one fifth (12) that of ethanol. Folacin may play an important role in the metabolism of methanol by catalyzing the elimination of formic acid (41). If this process proves to be as protective for humans as has been shown in other organisms (50, 38) it may account, in part, for the tremendous variability of human responses to acute methanol toxicity. Folacin is a nutrient often found lacking in the normal human diet, particularly during pregnancy and lactation (14)." It is well known that our primate ancestors were highly adapted to a diet of fruits and vegetables, and thus had the enzyme systems to prevent toxicity from the inevitable methanol content and from methanol from the degradation of pectins by bacteria in the colon. Humans have been living in groups in lifelong close proximity with fire and smoke, a potent source of formaldehyde, for about 2 million years. Fire has ever since been essential for survival, as has been intimate enclosed group living, especially as homo erectus, Neanderthals, and moderns successively adapted to very cold habitats. Yet formaldehyde is among the most potent of toxins, and cumulative to boot. Yet intimate enclosed group living in a variety of environments promotes extreme exposure to a variety of contagious, infectious diseases. And yet, humans lack two enzymes that protect against Methanol Chain Compounds toxicities. What is an obvious evolutionary explanation for this? MCC toxicities must serve to prevent and treat contagious infections from bacteria, fungi, parasites, and possibly viruses. This would generate a potent positive selection pressure to cause humans to evolve the ability to have increased MCC exposures, and to be multiply adapted to tolerate MC toxicities. Wine and beer serve throughout history to protect against water bourne pathogens. For centuries formaldehyde has been used to protect medical scientists from highly infected cadaver tissues. What is the scientific literature about MCC and the various groups of contagious infectious agents? It is a testable hypothesis as to whether MCC in many types of people, with the inevitable complex variations of genetics and diet, impede many simple infectious agents more than they harm critical body processes in complex human cells. It may be that MCC are important unexamined co-factors that strongly affect research and treatment of many infectious diseases. We might find, for instance, that in many humans some infections cause reduction of folic acid or folate levels, and thus increased MCC levels. Many traditional societies treat diseases with exposure to smoke, whether in a hut with a wood fire, or in a temple with incense. Likewise, alcohol drinks have been widely used as remedies. Did the daily ration of grog in the British Navy serve to reduce infections in the close and dirty confines of life on wooden ships? In the trenches of World War I, the British also had a daily ration -- were their rates of infection lower than for troops that had little liquor? These questions throw an entirely new light, expansive, tantalizing, and unifying, on the often contentious and poorly researched issues of MCC toxicity, especially the aspartame controversy. It would also be ironic, but typical of the actual complex evolution of science, for tobacco and wood smoke to be shown to have some benefits for infectious diseases. http://groups.yahoo.com/group/aspartameNM/message/1140 EPA Preliminary Remedial Goals, PRGs, 2003 Oct, air and tap water -- methanol, formaldehyde, formic acid -- not mentioned is methanol from aspartame, dark wines and liquors: Murray 2004.11.20 rmforall http://groups.yahoo.com/group/aspartameNM/message/1141 Nurses Health Study can quickly reveal the extent of aspartame (methanol, formaldehyde, formic acid) toxicity: Murray 2004.11.21 rmforall The Nurses Health Study is a bonanza of information about the health of probably hundreds of nurses who use 6 or more cans daily of diet soft drinks -- they have also stored blood and tissue samples from their immense pool of subjects. Dark wines and liquors, as well as aspartame, provide similar levels of methanol, above 100 mg daily, for long-term heavy users. Methanol is inevitably largely turned into formaldehyde, and thence largely into formic acid. Both products are toxic, and at this level of use, about 2 L daily, almost six 12-oz cans of diet drink, are above recent lifetime EPA [ PRG ] safety limits in tap water for methanol and formaldehyde of respectively, for a 60 kg person, 30 mg and 9 mg daily. The immediate health effects for dark wines and liquors are the infamous "morning after" hangover, for which many informed experts cite as the major cause the conversion of the methanol impurity, over one part in ten thousand (red wine has 128 mg/L methanol), into formaldehyde and formic acid. Everyone knows the complex progression of symptoms at this level of long-term, chronic toxicity. Aspartame reactors have a very similar progression. If 1% of all people exposed to alcohol and/or aspartame are heavy users with symptoms, then there would easily be about 2 million cases in the USA alone. This is a public health emergency. At the very least, professionals and the public should be alerted to investigate their own exposure, and be given a chance to try a very safe, simple, inexpensive treatment for complex, intractable, progressive symptoms -- reducing or eliminating their intake. There are as well, many safe substances that prevent or treat the toxicities -- for example, high folic acid levels expedite the elimination of formaldehyde. These toxicities are largely uncontrolled co-factors that affect every disease and must confuse and impede many health research programs on all levels. People in high-pressure, critical occupations, such as pilots, nuclear plant operators, and national leaders, should certainly be alerted. Also, two careful studies show substantial methanol release from degradation of pectins by bacteria in the colon from fruits and vegetables -- a topic that deserves careful, thorough research. Due to my bias, based on detailed reviews by Monte WC (1984) and by Mark D. Gold (2003), for months I have been discounting the startlingly high methanol levels reported in the abstract for Lindinger W (1997). I have been reducing the values in their abstract from g to mg, an unwarrented "correction" by a factor of a thousand, only to find that the full text study and their many related studies supply expert, robust results: Alcohol Clin Exp Res. 1997 Aug; 21(5): 939-43. Endogenous production of methanol after the consumption of fruit. Lindinger W, Taucher J, Jordan A, Hansel A, Vogel W. Institut fur Ionenphysik, Leopold Franzens Universitat Innsbruck, Austria. After the consumption of fruit, the concentration of methanol in the human body increases by as much as an order of magnitude. This is due to the degradation of natural pectin (which is esterified with methyl alcohol) in the human colon. In vivo tests performed by means of proton-transfer-reaction mass spectrometry show that consumed pectin in either a pure form (10 to 15 g) or a natural form (in 1 kg of apples) induces a significant increase of methanol in the breath (and by inference in the blood) of humans. The amount generated from pectin (0.4 to 1.4 g) is approximately equivalent to the total daily endogenous production (measured to be 0.3 to 0.6 g/day) or that obtained from 0.3 liters of 80-proof brandy (calculated to be 0.5 g). [ 1667 mg methanol per liter of brandy ] This dietary pectin may contribute to the development of nonalcoholic cirrhosis of the liver. PMID: 9267548 Alcohol Clin Exp Res. 1995 Oct; 19(5): 1147-50. Methanol in human breath. Taucher J, Lagg A, Hansel A, Vogel W, Lindinger W. Institut fur Ionenphysik, Universitat Innsbruck, Austria. Using proton transfer reaction-mass spectrometry for trace gas analysis of the human breath, the concentrations of methanol and ethanol have been measured for various test persons consuming alcoholic beverages and various amounts of fruits, respectively. The methanol concentrations increased from a natural (physiological) level of approximately 0.4 ppm up to approximately 2 ppm a few hours after eating about 1/2 kg of fruits, and about the same concentration was reached after drinking of 100 ml brandy containing 24% volume of ethanol and 0.19% volume of methanol. [ 24 ml = 61 g ethanol, and 0.19 ml = 0.34 g = 340 mg methanol ] PMID: 8561283 I urge Channing Laboratory and its participating universities to rapidly mount an in-house study to study the Nurses Health Study database for the hundreds of nurses who are long-term users, above 6 cans diet drinks daily, for correlations with every disease, as well as ubiquitous co-factors like wine and liquor, cigarette smoke, and fruits and vegetables. It could vastly serve the world public health to make the initial findings widely available immediately. The disparaged issue of aspartame toxicity could be swiftly made legitimate, and the resulting progress on all levels remarkably accelerated. A single scientist could do this. Comments pro and con are welcome. A convenient venue would be the newsgroup: bionet.toxicology. Rich Murray, MA Room For All rmforall@comcast.net 1943 Otowi Road, Santa Fe, New Mexico 87505 USA 505-501-2298 http://groups.yahoo.com/group/aspartameNM/messages 137 members, 1,142 posts in a public searchable archive ************************************************************** http://groups.yahoo.com/group/aspartameNM/message/957 safety of aspartame Part 1/2 12.4.2: EC HCPD-G SCF: Murray 2003.01.12 rmforall EU Scientific Committee on Food, a whitewash http://groups.yahoo.com/group/aspartameNM/message/1045 http://www.holisticmed.com/aspartame/scf2002-response.htm Mark Gold exhaustively critiques European Commission Scientific Committee on Food re aspartame ( 2002.12.04 ): 59 pages, 230 references "C. Public Relations, Aspartame, Methanol, and Formaldehyde Before we discuss what little the Committee did say related to aspartame and formaldehyde, it is important to answer all of the typical public relations statements from the manufacturer and their consultants who claim there is no problem with aspartame and formaldehyde. The answers provided below will be brief. Much more detailed and referenced answers can be found at ATIC (2001) on the Internet at: [ http://www.holisticmed.com/aspartame/abuse/methanol.html ]. Chart of Aspartame Manufacturer Public Relations Statements Related to Methanol and Formaldehyde Manufacturer Claim --- Independent Response Methanol is found in fruits and alcoholic beverages at higher levels than in aspartame products. --- Alcoholic beverages contain large amounts of ethanol (a protective factor) which allows methanol to be excreted before much of it is converted into formaldehyde (Leaf 1952, Liesivuori 1991, Roe 1982). Fruit juices have protective factors as well that prevent formaldehyde poisoning. Fruit juices produce enough methanol to "qualify as significantly methanol-contaminated liquor" (Lindinger 1997) -- more methanol than what causes chronic health problems in occupational exposure (Kazeniac 1970, Kavet 1990, Frederick 1984, Kingsley 1954-55). Since we do not see chronic poisoning from fruit juices, they must contain protective factors as well. Fruit juices have ethanol as well as other possible protective factors." http://groups.yahoo.com/group/aspartameNM/message/870 Aspartame: Methanol and the Public Interest 1984: Monte: Murray 2002.09.23 rmforall Dr. Woodrow C. Monte Aspartame: methanol, and the public health. Journal of Applied Nutrition 1984; 36 (1): 42-54. (62 references) Professsor of Food Science [retired 1992] Arizona State University, Tempe, Arizona 85287 woodymonte@xtra.co.nz *********************************************************** [ Comments by Rich Murray are in square bracketts. Without changing text, except for omitting long equations, figures, and tables, spacing has been added to give emphasis and increase readability. Following are some specific extracts. ] "That substantial amounts of methanol metabolites or by-products are retained for a long time is verified by Horton et al. (1992) who estimated that 18 h following an iv injection of 100 mg/kg of 14C-methanol in male Fischer-344 rats, only 57% of the dose was eliminated from the body. From the data of Dorman et al. (1994) and Medinsky et al. (1997), it can further be calculated that 48 h following the start of a 2-h inhalation exposure to 900 ppm of 14C-methanol vapors in female cynomolgus monkeys, only 23% of the absorbed 14C-methanol was eliminated from the body. These findings are corroborated by the data of Heck et al. (1983) showing that 40% of a 14C-formaldehyde inhalation dose remained in the body 70 h postexposure." "Exposure to methanol also results from the consumption of certain foodstuffs (fruits, fruit juices, certain vegetables, aspartame sweetener, roasted coffee, honey) and alcoholic beverages (Health Effects Institute, 1987; Jacobsen et al., 1988)." [ It's unusual for a mainstream journal article to mention "aspartame sweetener" and "alcoholic beverages" to be methanol sources. "However, the severe toxic effects are usually associated with the production and accumulation of formic acid, which causes metabolic acidosis and visual impairment that can lead to blindness and death at blood concentrations of methanol above 31 mmol/l (Røe, 1982; Tephly and McMartin, 1984; U.S. DHHS, 1993). Although the acute toxic effects of methanol in humans are well documented, little is known about the chronic effects of low exposure doses, which are of interest in view of the potential use of methanol as an engine fuel and current use as a solvent and chemical intermediate. Gestational exposure studies in pregnant rodents (mice and rats) have also shown that high methanol inhalation exposures (5000 or 10,000 ppm and more, 7 h/day during days 6 or 7 to 15 of gestation) can induce birth defects (Bolon et al., 1993; IPCS, 1997; Nelson et al., 1985)." "The corresponding average elimination half-life of absorbed methanol through metabolism to formaldehyde was estimated to be 1.3, 0.7-3.2, and 1.7 h." [ This shows for ingested methanol from the readily released 11% methanol component of aspartame diet sodas, that by three half-lives, 3 X 1.7 h = 5.1 hr, 7/8 = 88 % of the methanol would be substantially turned into formaldehyde. In dark wines and liquors, the conversion of methanol impurity, about one part in ten thousand, into formaldehyde and then formic acid is prevented for many hours, as the responsible enzyme is taken up by the remaining ethanol. When after many hours all the ethanol is metabolized, the conversion of the remaining methanol into formaldehyde and formic acid is the major cause of the many difficult symtoms of "morning after" hangover. ] "Inversely, in monkeys and in humans, a larger fraction of body burden of formaldehyde is rapidly transferred to a long-term component. The latter represents the formaldehyde that (directly or after oxidation to formate) binds to various endogenous molecules..." "Animal studies have reported that systemic methanol is eliminated mainly by metabolism (70 to 97% of absorbed dose) and only a small fraction is eliminated as unchanged methanol in urine and in the expired air (< 3-4%) (Dorman et al., 1994; Horton et al., 1992). Systemic methanol is extensively metabolized by liver alcohol dehydrogenase and catalase-peroxidase enzymes to formaldehyde, which is in turn rapidly oxidized to formic acid by formaldehyde dehydrogenase enzymes (Goodman and Tephly, 1968; Heck et al., 1983; Røe, 1982; Tephly and McMartin, 1984). Under physiological conditions, formic acid dissociates to formate and hydrogen ions. Current evidence indicates that, in rodents, methanol is converted mainly by the catalase-peroxidase system whereas monkeys and humans metabolize methanol mainly through the alcohol dehydrogenase system (Goodman and Tephly, 1968; Tephly and McMartin, 1984). Formaldehyde, as it is highly reactive, forms relatively stable adducts with cellular constituents (Heck et al., 1983; Røe, 1982)." "The whole body loads of methanol, formaldehyde, formate, and unobserved by-products of formaldehyde metabolism were followed. Since methanol distributes quite evenly in the total body water, detailed compartmental representation of body tissue loads was not deemed necessary." "According to model predictions, congruent with the data in the literature (Dorman et al., 1994; Horton et al., 1992), a certain fraction of formaldehyde is readily oxidized to formate, a major fraction of which is rapidly converted to CO2 and exhaled, whereas a small fraction is excreted as formic acid in urine. However, fits to the available data in rats and monkeys of Horton et al. (1992) and Dorman et al. (1994) show that, once formed, a substantial fraction of formaldehyde is converted to unobserved forms. This pathway contributes to a long-term unobserved compartment. The latter, most plausibly, represents either the formaldehyde that (directly or after oxidation to formate) binds to various endogenous molecules (Heck et al., 1983; Røe, 1982) or is incorporated in the tetrahydrofolic-acid-dependent one-carbon pathway to become the building block of a number of synthetic pathways (Røe, 1982; Tephly and McMartin, 1984). That substantial amounts of methanol metabolites or by-products are retained for a long time is verified by Horton et al. (1992) who estimated that 18 h following an iv injection of 100 mg/kg of 14C-methanol in male Fischer-344 rats, only 57% of the dose was eliminated from the body. From the data of Dorman et al. (1994) and Medinsky et al. (1997), it can further be calculated that 48 h following the start of a 2-h inhalation exposure to 900 ppm of 14C-methanol vapors in female cynomolgus monkeys, only 23% of the absorbed 14C-methanol was eliminated from the body. These findings are corroborated by the data of Heck et al. (1983) showing that 40% of a 14C-formaldehyde inhalation dose remained in the body 70 h postexposure. In the present study, the model proposed rests on acute exposure data, where the time profiles of methanol and its metabolites were determined only over short time periods (a maximum of 6 h of exposure and a maximum of 48 h postexposure). This does not allow observation of the slow release from the long-term components. It is to be noted that most of the published studies on the detailed disposition kinetics of methanol regard controlled short-term (iv injection or continuous inhalation exposure over a few hours) methanol exposures in rats, primates, and humans (Batterman et al., 1998; Damian and Raabe, 1996; Dorman et al., 1994; Ferry et al., 1980; Fisher et al., 2000; Franzblau et al., 1995; Horton et al., 1992; Jacobsen et al., 1988; Osterloh et al., 1996; Pollack et al., 1993; Sedivec et al., 1981; Ward et al., 1995; Ward and Pollack, 1996). Experimental studies on the detailed time profiles following controlled repeated exposures to methanol are lacking." "Thus, in monkeys and plausibly humans, a much larger fraction of body formaldehyde is rapidly converted to unobserved forms rather than passed on to formate and eventually CO2." "However, the volume of distribution of formate was larger than that of methanol, which strongly suggests that formate distributes in body constituents other than water, such as proteins. The closeness of our simulations to the available experimental data on the time course of formate blood concentrations is consistent with the volume of distribution concept (i.e., rapid exchanges between the nonblood pool of formate and blood formate)." "Also, background concentrations of formate are subject to wide interindividual variations (Baumann and Angerer, 1979; D'Alessandro et al., 1994; Franzblau et al., 1995; Heinrich and Angerer, 1982; Lee et al., 1992; Osterloh et al., 1996; Sedivec et al., 1981)." [ There's an on-going debate as to how much of methanol toxicity and genotoxicity is due to its formaldehyde or formic acid products, along with a dearth of evidence about the actual biochemical disposition in specific tissues of people exposed long-term to chronic doses, as in the case of alcoholics or aspartame reactors. Fully 11% of aspartame is methanol -- 1,120 mg aspartame in 2 L diet soda, almost six 12-oz cans, gives 123 mg methanol (wood alcohol). However, about 30% of the methanol remains in the body as cumulative durable toxic metabolites of formaldehyde and formic acid, 37 mg daily, a gram every month, accumulating in and affecting every tissue. If only 10% of the methanol accumulates daily as formaldehyde, that would give 12 mg daily formaldehyde accumulation -- about 60 times more than the 0.2 mg from 10% retention of the 2 mg EPA daily limit for formaldehyde in drinking water. Bear in mind that the EPA limit for formaldehyde in drinking water is 1 ppm, or 2 mg daily for a typical daily consumption of 2 L of water. http://groups.yahoo.com/group/aspartameNM/message/835 ATSDR: EPA limit 1 ppm formaldehyde in drinking water July 1999: Murray 2002.05.30 rmforall This is the same limit published May 2, 2002 for California. http://groups.yahoo.com/group/aspartameNM/message/1108 faults in 1999 July EPA 468-page formaldehyde profile: Elzbieta Skrzydlewska PhD, Assc. Prof., Medical U. of Bialystok, Poland, abstracts -- ethanol, methanol, formaldehyde, formic acid, acetaldehyde, lipid peroxidation, green tea, aging, Lyme disease: Murray 2004.08.08 rmforall Herein I offer abstracts and three full texts of dozens of studies by a world-class biochemist and her associates, mostly experiments with rats, on ethanol toxicity since 1984 and methanol toxicity since 1993. Enough details are provided to show the competency and credibility of E. Skrzydlewska and her colleagues over two decades, and to make access to their literature more convenient for professionals. It is important that many of her studies suggest that many safe substances may prevent or treat toxicity from methanol and its inevitable toxic human body products, formaldehyde and formic acid: N-acetylcysteine (2000); U-83836E containing a trolox ring (1997); green tea (2004); vitamins E, C, A, and beta-carotene (2004); glutathione (2001); N-Acetylcysteine (NAC) (2001); melatonin (2001); low and medium levels of cysteine (1990). http://taylorandfrancis.metapress.com/openurl.asp?genre=article&eissn=1537-6524&volume=13&issue=4&spage=277 Toxicology Mechanisms and Methods Publisher: Taylor & Francis Health Sciences, part of the Taylor & Francis Group Issue: Volume 13, Number 4 / Oct-Dec 2003 Pages: 277 - 293 Toxicological and Metabolic Consequences of Methanol Poisoning Elzbieta Skrzydlewska, Assoc. Professor, MSc, PhD, Deputy Dean of Faculty of Pharmacy, Head of Department of Analytical Chemistry, Medical University of Bialystok, Mickiewicza 2A 15-230 Bialystok 8, P.O. Box 14, Poland skrzydle@amb.ac.bialystok.pl http://www.amb.edu.pl/en/sites/university.html dzss@amb.edu.pl Kilinskiego 1 15-089 Bialystok, Poland fax (48 85)7485408 Abstract: Methanol, when introduced into all mammals, is oxidized into formaldehyde and then into formate, mainly in the liver. Such metabolism is accompanied by the formation of free radicals. In all animals, methanol oxidation, which is relatively slow, proceeds via the same intermediary stages, usually in the liver, and various metabolic systems are involved in the process, depending on the animal species. In nonprimates, methanol is oxidized by the catalase-peroxidase system, whereas in primates, the alcohol dehydrogenase system takes the main role in methanol oxidation. The first metabolite (formaldehyde is rapidly oxidized by formaldehyde dehydrogenase) is the reduced glutathione (GSH)-dependent enzyme. Generated formic acid is metabolized into carbon dioxide with the participation of H4folate and two enzymes, 10-formyl H4folate synthetase and dehydrogenase, whereas nonprimates oxidize formate efficiently. Humans and monkeys possess low hepatic H4folate and 10-formyl H4folate dehydrogenase levels and are characterized by the accumulation of formate after methanol intoxication. The consequences of methanol metabolism and toxicity distinguish the human and monkey from lower animals. Formic acid is likely to be the cause of the metabolic acidosis and ocular toxicity in humans and monkeys, which is not observed in most lower animals. Nevertheless, chemically reactive formaldehyde and free radicals may damage most of the components of the cells of all animal species, mainly proteins and lipids. The modification of cell components results in changes in their functions. Methanol intoxication provokes a decrease in the activity and concentration of antioxidant enzymatic as well as nonenzymatic parameters, causing enhanced membrane peroxidation of phospholipids. The modification of protein structure by formaldehyde as well as by free radicals results changes in their functions, especially in the activity of proteolytic enzymes and their inhibitors, which causes disturbances in the proteolytic-antiproteolytic balance toward the proteolytics and enhances the generation of free radicals. Such a situation can lead to destructive processes because components of the proteolytic-antiproteolytic system during enhanced membrane lipid peroxidation may penetrate from blood into extracellular space, and an uncontrolled proteolysis can occur. This applies particularly to extracellular matrix proteins. Keywords: Free Radicals, Methanol Metabolism, Methanol Poisoning, Proteases, Protease Inhibitors ] ************************************************************* http://www.toxsci.oupjournals.org/cgi/content/full/64/2/169 Toxicological Sciences 64, 169-184 (2001) Copyright © 2001 by the Society of Toxicology BIOTRANSFORMATION AND TOXICOKINETIC A Biologically Based Dynamic Model for Predicting the Disposition of Methanol and Its Metabolites in Animals and Humans Michèle Bouchard *, #,1, bouchmic@magellan.umontreal.ca Robert C. Brunet, # brunet@dms.umontreal.ca Pierre-Olivier Droz, # and Gaétan Carrier* gaetan.carrier@umontreal.ca * Department of Environmental and Occupational Health, Faculty of Medicine, Université de Montréal, P.O. Box 6128, Main Station, Montréal, Québec, Canada, H3C 3J7; # Institut Universitaire romand de Santé au Travail, rue du Bugnon 19, CH-1005, Lausanne, Switzerland, and # Département de Mathématiques et de Statistique and Centre de Recherches Mathématiques, Faculté des arts et des sciences, Université de Montréal, P.O. Box 6128, Main Station, Montréal, Québec, Canada, H3C 3J7 NOTES 1 To whom correspondence should be addressed at Département de santé environnementale et santé au travail, Université de Montréal, P.O. Box 6128, Main Station, Montréal, Québec, H3C 3J7, Canada. Fax: (514) 343-2200. E-mail: bouchmic@magellan.umontreal.ca Received May 10, 2001; accepted August 28, 2001 ABSTRACT TOP ABSTRACT INTRODUCTION METHOD AND MODEL PRESENTATION RESULTS DISCUSSION APPENDIX REFERENCES A multicompartment biologically based dynamic model was developed to describe the time evolution of methanol and its metabolites in the whole body and in accessible biological matrices of rats, monkeys, and humans following different exposure scenarios. The dynamic of intercompartment exchanges was described mathematically by a mass balance differential equation system. The model's conceptual and functional representation was the same for rats, monkeys, and humans, but relevant published data specific to the species of interest served to determine the critical parameters of the kinetics. Simulations provided a close approximation to kinetic data available in the published literature. The average pulmonary absorption fraction of methanol was estimated to be 0.60 in rats, 0.69 in monkeys, and 0.58-0.82 in human volunteers. The corresponding average elimination half-life of absorbed methanol through metabolism to formaldehyde was estimated to be 1.3, 0.7-3.2, and 1.7 h. Saturation of methanol metabolism appeared to occur at a lower exposure in rats than in monkeys and humans. Also, the main species difference in the kinetics was attributed to a metabolism rate constant of whole body formaldehyde to formate estimated to be twice as high in rats as in monkeys. Inversely, in monkeys and in humans, a larger fraction of body burden of formaldehyde is rapidly transferred to a long-term component. The latter represents the formaldehyde that (directly or after oxidation to formate) binds to various endogenous molecules or is taken up by the tetrahydrofolic-acid-dependent one-carbon pathway to become the building block of synthetic pathways. This model can be used to quantitatively relate methanol or its metabolites in biological matrices to the absorbed dose and tissue burden at any point in time in rats, monkeys, and humans for different exposures, thus reducing uncertainties in the dose-response relationship, and animal-to-human and exposure scenario comparisons. The model, adapted to kinetic data in human volunteers exposed acutely to methanol vapors, predicts that 8-h inhalation exposures ranging from 500 to 2000 ppm, without physical activities, are needed to increase concentrations of blood formate and urinary formic acid above mean background values reported by various authors (4.9-10.3 and 6.3-13 mg/liter, respectively). This leaves blood and urinary methanol concentrations as the most sensitive biomarkers of absorbed methanol. Key Words: methanol; formaldehyde; formate; toxicokinetics; modeling; animals; humans. Methanol is widely used as an industrial solvent and chemical intermediate (Kavet and Nauss, 1990). It has also received serious consideration as an alternative automotive fuel or fuel additive (Health Effects Institute, 1987). Inhalation is a major route of human exposure to methanol in the occupational and general environments although skin exposure can occur in certain industrial settings (Baumann and Angerer, 1979; Downie et al 1992; Heinrich and Angerer, 1982; Kawai et al., 1991). Exposure to methanol also results from the consumption of certain foodstuffs (fruits, fruit juices, certain vegetables, aspartame sweetener, roasted coffee, honey) and alcoholic beverages (Health Effects Institute, 1987; Jacobsen et al., 1988). The toxic effects of acute exposures to high methanol doses in humans are well documented (Liesivuori and Savolainen, 1991; Røe, 1982; Tephly and McMartin, 1984; U.S. DHHS, 1993). Neurological effects, such as the initial transient depression of the central nervous system, have generally been reported at blood concentrations of methanol above 6 mmol/l (U.S. DHHS, 1993). However, the severe toxic effects are usually associated with the production and accumulation of formic acid, which causes metabolic acidosis and visual impairment that can lead to blindness and death at blood concentrations of methanol above 31 mmol/l (Røe, 1982; Tephly and McMartin, 1984; U.S. DHHS, 1993). Although the acute toxic effects of methanol in humans are well documented, little is known about the chronic effects of low exposure doses, which are of interest in view of the potential use of methanol as an engine fuel and current use as a solvent and chemical intermediate. Gestational exposure studies in pregnant rodents (mice and rats) have also shown that high methanol inhalation exposures (5000 or 10,000 ppm and more, 7 h/day during days 6 or 7 to 15 of gestation) can induce birth defects (Bolon et al., 1993; IPCS, 1997; Nelson et al., 1985). The potential deleterious effects of methanol have prompted extensive research on its uptake and disposition in animals and humans. This has led to the findings that pulmonary absorption of methanol is very rapid and absorption fraction ranges from about 60 to 85% depending on the species (Dorman et al., 1994; Fisher et al., 2000; Horton et al., 1992). Due to the high water solubility of methanol, the distribution of absorbed methanol in the tissues of the body is a function of their relative water content (Sejersted et al., 1983). Animal studies have reported that systemic methanol is eliminated mainly by metabolism (70 to 97% of absorbed dose) and only a small fraction is eliminated as unchanged methanol in urine and in the expired air (< 3-4%) (Dorman et al., 1994; Horton et al., 1992). Systemic methanol is extensively metabolized by liver alcohol dehydrogenase and catalase-peroxidase enzymes to formaldehyde, which is in turn rapidly oxidized to formic acid by formaldehyde dehydrogenase enzymes (Goodman and Tephly, 1968; Heck et al., 1983; Røe, 1982; Tephly and McMartin, 1984). Under physiological conditions, formic acid dissociates to formate and hydrogen ions. Current evidence indicates that, in rodents, methanol is converted mainly by the catalase-peroxidase system whereas monkeys and humans metabolize methanol mainly through the alcohol dehydrogenase system (Goodman and Tephly, 1968; Tephly and McMartin, 1984). Formaldehyde, as it is highly reactive, forms relatively stable adducts with cellular constituents (Heck et al., 1983; Røe, 1982). It can also enter, directly or after oxidation to formate, the tetrahydrofolic-acid-dependent one-carbon pathway to become the building block of many synthetic pathways (Røe, 1982; Tephly and McMartin, 1984). The detoxification of formate occurs mainly by a tetrahydrofolate-dependent multistep pathway to carbon dioxide (CO2) (McMartin et al., 1977; Palese and Tephly, 1975). A small percentage of body formate is also eliminated directly in the urine (Dorman et al., 1994; Horton et al., 1992). Marked species differences in methanol toxicity and metabolism have been reported. Primates and humans appear to be more susceptible to the acute toxicity of methanol than rodents (Tephly and McMartin, 1984). This has been mainly attributed to the slower metabolism and elimination rate of formate in larger species (Tephly and McMartin, 1984). Based on the available toxicokinetic data of methanol in rats, mice, monkeys, and humans, toxicokinetic processes were described in the past using classic 1 to 3 compartmental models with saturable elimination (Batterman et al., 1998; Damian and Raabe, 1996; Dorman et al., 1994; Nihlén and Droz, 2000; Pollack and Brouwer, 1996; Pollack et al., 1993; Ward et al., 1995; Ward and Pollack, 1996). Physiologically based pharmacokinetic (PBPK) models for methanol in animals and humans were also developed (Fisher et al., 2000; Horton et al., 1992; Perkins et al., 1995; Ward et al., 1997). Recently, a different type of multicompartment modeling approach has been developed to describe the disposition kinetics of polychlorinated dibenzo-dioxins and furans (PCDD and PCDFs) (Carrier et al., 1995a,b), azinphosmethyl and its alkylphosphate metabolites (Carrier and Brunet, 1999), and methyl mercury and its inorganic metabolites (Carrier et al., 2001a,b). This type of biologically based dynamic model is a refinement of classic compartment models, but is closer to biological processes and enables simulations for a variety of exposure scenarios in different species. This heuristic approach allows essential characteristics of intercompartmental transfer processes to be captured using a minimum of parameters and without the need for extensive knowledge of all the physiological processes. The ultimate goal of this approach is to develop a robust human toxicokinetic model based on human data, thus avoiding as much as possible uncertainties associated with animal to human extrapolations. The objective of the present study was to develop and validate such a biologically based dynamic model to describe the time evolution of methanol and its metabolites in the whole body, and in accessible biological matrices (blood, urine, and expired air), and allow links to be made between the different compartments. This model is constructed by establishing the overall biological determinants of methanol disposition in animals and humans, taking into account the different time-scales involved in the biological processes. The model parameters specific to the species of interest are then determined from direct fits to the in vivo time course data of methanol and its metabolites in blood and excreta (urine and expired air), available in the published literature. Model Development A toxicokinetic, biologically based, dynamic model was developed to describe the time evolution of methanol biodisposition in the animal (rat and monkey) and human body. The modeling process can be described in four steps: (1) the conceptual and functional representation of the model, (2) the determination of parameters, (3) the simulation of the kinetic profile, and (4) the validation of the model. Conceptual and functional representation. The disposition kinetics of methanol and its metabolites following exposure to methanol was modeled using a multicompartment dynamic system, described mathematically by a system of coupled differential equations. The model conceptual and functional representation is depicted in Figure 1. It aims to be sufficiently detailed to describe the available in vivo data provided by Horton et al. (1992) on the disposition kinetics of methanol and its metabolites in rats. It was then verified that it described equally well the monkey and human kinetic behavior (Dorman et al., 1994; Osterloh et al., 1996; Sedivec et al., 1981). View larger version (30K): [in this window] [in a new window] FIG. 1. Conceptual representation of methanol kinetics. Symbols are described in Table 1. The whole body (blood and tissues) and the excretory routes (urine and exhaled air) were each represented by a compartment. The whole body loads of methanol, formaldehyde, formate, and unobserved by-products of formaldehyde metabolism were followed. Since methanol distributes quite evenly in the total body water, detailed compartmental representation of body tissue loads was not deemed necessary. Formaldehyde in the whole body was also represented as a separate compartment although its metabolism rate is too rapid to allow its quantification (half-life of about 1.5 min according to McMartin et al. [1979] and Tephly and McMartin [1984]). It can be shown that, under such fast breakdown, only formaldehyde partitioning between formate and other by-products is relevant to the unfolding of the dynamics. The respiratory tract was further represented as a separate compartment since it is the route of entry of inhaled methanol. Excretion compartments were the methanol in the exhaled air, the urinary methanol, the urinary formic acid, the CO2 in the exhaled air, and the excreted unobserved metabolites. The dynamic of intercompartment exchanges was then described mathematically by a mass balance differential equation system (see Appendix). Essentially, the rates of change in the amounts of methanol and its metabolites in a given compartment were described as the difference between compartment rates of uptake and loss. (Symbols used in the functional representation of the model are presented in Table 1.) Solving numerically the system of differential equations yielded the time courses of methanol and its metabolites in the different compartments. View this table: [in this window] [in a new window] TABLE 1 Symbols Used in the Conceptual and Functional Representation of the Model It should also be mentioned that metabolism was considered to follow Michaelis-Menten kinetics. However, only in the case of methanol metabolism to formaldehyde was a saturation constant introduced since, with the exposure dose range used in the studies on which the model is based, no saturation of formate or CO2 metabolism was apparent (Dorman et al., 1994; Horton et al., 1992; Osterloh et al., 1996 Sedivec et al., 1981). Determination of the parameters. Unknown parameters were estimated individually from a statistical best-fit to the experimental data specific to the species of interest, by using the explicit solutions of subsystems of differential equations when possible (see Appendix). A professional edition of a MathCad software was used for this purpose (MathSoft Inc., Cambridge, MA). Rat parameters. Parameters to be determined were the intercompartment transfer rate coefficients and metabolism rate constants. Data of Horton et al. (1992) in male Fischer-344 rats exposed to a single iv dose of 100 mg per kg of body weight of 14C-methanol (n = 4) were used to determine the rat parameters. Blood concentration-time profiles (expressed in mg/l) and cumulative urinary excretion time courses of 14C-methanol and 14C-formate (expressed in µmol) were determined by these authors as well as the cumulative exhalation time courses of 14C-methanol and 14CO2 (expressed in µmol). In the current study, for the fitting of experimental data and to determine parameters, all the experimental values were converted to burdens expressed in moles. It was then verified that the mass balance was maintained at all time points. Also, reported blood concentration values were converted to whole body burdens by multiplication by the apparent volume of distribution (Vd). In rats, the apparent volume of distribution of methanol (VdMeOH) was determined so that the initial experimental concentration of methanol in blood at time t = 5 min, when converted in terms of burden, gives the iv dose (700 µmol) reported by Horton et al. (1992). Monkey parameters. To adapt the model to monkey data, only the values of the intercompartment transfer rates and metabolism constants needed to be modified. Using the same approach as in rats, transfer parameters values of the general model solutions were estimated individually by best-fits, using MathCad, to the available experimental data of Dorman et al. (1994). These authors exposed 4 adult female cynomolgus monkeys (Macaca fascicularis, 3-5.5 kg) by inhalation to 900 ppm of 14C-methanol for 2 h. Blood concentration-time profiles of 14C-methanol and 14C-formate (expressed in µmol/l) were determined as well as the cumulative urinary excretion of 14C-methanol and 14C-formate 48-h postexposure (expressed in µmol). The time courses of 14C-methanol and 14CO2 exhalation rates (µmol/min) were also established. For the determination of the parameter values, the latter rates were converted to cumulative excretion. The pulmonary ventilation rate of female cynomolgus monkeys used in the model was that reported by Dorman et al. (1994), that is on average 33 l/h or 0.56 l/min (equivalent to 0.033 m3/h or 0.8 m3/day). In monkeys, the apparent volume of distribution of methanol was calculated by best-fit of the following equation to the data observed during the constant inhalation built up of methanol blood concentration (B(t)): [ long equation ] where kelim is the sum of all rates of methanol elimination (metabolism, exhalation, and urinary excretion). Human parameters. When possible, the constants were determined using the available human data. This includes the pulmonary absorption fraction of methanol, the pulmonary ventilation rate, the apparent volume of distribution of methanol, the metabolism rate constant kmet of whole body methanol to formaldehyde, and the transfer rate constant km of whole body methanol to urine. The other constant parameters were left as determined in monkeys, which are considered as good surrogates to humans for the study of methanol kinetics. Pulmonary absorption fraction of methanol used in the model adapted to humans was that reported by Sedivec et al. (1981). The human value was nonetheless close to that determined in rats and monkeys. Human pulmonary ventilation rate used in the model was that reported by Sedivec et al. (1981), that is on average 10.8 l/min. The apparent volume of distribution of methanol was that reported in the literature, hence, corresponds to the volume of human body fluids (liters), expressed per kilogram of body weight. This value is about the same as that determined using the experimental monkey data. The constant parameter kmet was determined from a best-fit to the blood concentration-time profile of methanol in human volunteers exposed to 200 ppm of methanol vapors for 4 h, as determined by Osterloh et al. (1996). The km value was determined by adjustment to the data of Sedivec et al. (1981) on the urinary excretion time course curves of methanol in volunteers during and following an 8-h inhalation exposure to 300 mg/m3 of methanol vapors. The experimental data of Sedivec et al. (1981) on the time evolution of urinary methanol concentrations were converted to cumulative urinary excretion of methanol (in µmol) by considering an average time-dependent fraction of a daily urinary excretion of 1.5 l (Knuiman et al., 1986). Model simulation. Once the parameters were determined individually by statistical fits to the experimental data, mathematical resolution of the complete model, as represented by the system of differential equations, was performed by the numerical Runge-Kutta method. Model resolution and simulations were also conducted using Mathcad software. This allows prediction of the time evolution of methanol and its metabolites in the different model compartments. In the model, the exposure dose was converted in µmoles for both the iv and inhalation exposures. Thus, whole body burdens and amounts excreted in urine and in the exhaled air are first expressed in µmoles. In order to simulate the blood concentrations of methanol or formate as a function of time, the amounts in the whole body predicted by the model were simply divided by the respective apparent volume of distribution. For rats and monkeys, the apparent volume of distribution of formate (VdFA) was estimated using a conservation of mass equation for formate burden, and by a best-fit to the observed time course of experimental blood concentration values of formate. For monkeys, this amounts to 6 times the apparent volume of distribution of methanol. For humans, the same multiple was used. To simulate the concentration-time profile of methanol in urine, predicted excretion rates (dM(t)/dt = km x X(t), expressed in µmol/min) were divided by the urinary flow rate (l/min). To simulate the concentration-time profile of methanol in the exhaled air, predicted exhalation rates (dE(t)/dt = kre x L(t) + kex x X(t), expressed in µmol/min) were divided by the pulmonary ventilation rate (m3/min). Simulations of exposure scenarios, where continuous or intermittent doses are administered through time, were performed by introducing a nonhomogenous term, g(t), describing these time varying inputs (see Appendix). Simulations can also be conducted for different routes of exposure (iv, inhalation). Model Validation The model developed using the previously mentioned data was validated using a new set of experimental data. This includes the kinetic time profiles presented in the inhalation studies of Horton et al. (1992) in rats and monkeys and Batterman et al. (1998) in human volunteers. Also, some human data of Sedivec et al. (1981) not used in the development of the model served to validate the model. Validation using inhalation data of Horton et al. (1992) in rats. The model developed using the iv data of Horton et al. (1992) in rats was validated with the inhalation data of the same authors, on the blood concentration-time profile of methanol during and following 6-h inhalation exposures to 200, 1200, and 2000 ppm of methanol in male Fischer-344 rats (n = 4 per group). For those simulations, the average pulmonary ventilation rate used was 40 ml/min (equivalent to 0.0021 m3/h or 0.051 m3/day) for the 200 ppm dose, 40 ml/min (equivalent to 0.0024 m3/h or 0.058 m3/day) for the 1200 ppm dose, and 60 ml/min (equivalent to 0.0033 m3/h or 0.080 m3/day) for the 2000 ppm dose to obtain the best-fit to the experimental data as compared to the average value of 3.04 l/h or 50 ml/min (equivalent to 0.0030 m3/h or 0.073 m3/day) reported by Horton et al. (1992). Validation using inhalation data of Horton et al. (1992) in monkeys. The model adapted to the monkey data of Dorman et al. (1996) was validated using the data of Horton et al. (1992) on the blood concentration-time profile of methanol in 3 young adult male rhesus monkeys (Macaca mulatta, 5-7 kg) exposed to methanol vapor concentrations of 200, 1200, or 2000 ppm for 6 h. For these simulations, the average pulmonary ventilation rate was that reported by Horton et al. (1992), that is 48.9 l/h or 0.81 l/min (equivalent to 0.049 m3/h or 1.2 m3/day). Validation using inhalation data of Sedivec et al. (1981) and Batterman et al. (1998) in humans. The data of Sedivec et al. (1981) on the urinary excretion time course curves of methanol in volunteers during and following 8-h inhalation exposures to 102 and 205 mg/m3 of methanol vapors were used in the validation process of the model for humans. The model adapted to human data was also validated using the data of Batterman et al. (1998) on the time-dependent disposition of methanol in blood, urine, and breath of volunteers exposed to methanol vapor concentration of 800 ppm for periods of 0.5, 1, and 2 h. Batterman et al. (1998) presented their data as urinary and exhaled concentration-time profiles (expressed in mg/l and ppm, respectively). Although in this article the time courses of methanol cumulative excretion in urine and exhaled air are usually presented to insure mass balance conservation, it was also verified that the model gave a good prediction of the overall concentration-time profiles of methanol in urine and exhaled air (data not shown). To obtain a good fit on both the concentration values and cumulative burdens, a time-dependent fraction of a daily urinary excretion of 2.4 l for the 30 min and 2 h exposures and of 2.7 l for the 1 h exposure had to be considered. It has been reported that the daily personal urine volume may commonly vary from 0.6 l to more than 2.5 l (Knuiman et al., 1986). The average pulmonary ventilation rate used was 11.3, 8.4, and 10.8 l/min for the 30 min, 1 h, and 2 h exposures, respectively, to obtain a best-fit to the exhalation data. These latter rates are in the value range reported by Sedivec et al. (1981; average [range]: 10.8 [8.4-13.8] l/min). Model Developed Using the IV Data of Horton et al. (1992) in Male Fischer-344 Rats Table 2 presents the rat parameter values of the model determined using the data of Horton et al. (1992) in male Fischer rats exposed via iv to 100 mg of 14C-labeled methanol per kg of body weight (see Table 1 for the description of symbols). Figures 2 and 3 show that these parameter values allowed to reproduce closely the data presented by Horton et al. (1992) on the time courses of blood concentrations of methanol and formate as well as on the cumulative urinary excretion of methanol and formate and the cumulative exhalation of methanol and CO2. View this table: [in this window] [in a new window] TABLE 2 Numerical Values of Constant Parameters Used in the Model Adjusted to Male Fischer-344 rat, Female Cynomolgus Monkey, and Human Data View larger version (15K): [in this window] [in a new window] FIG. 2. Model simulations (lines) compared with experimental data of Horton et al. (1992) on the concentration-time courses of methanol (crossbars) and formate (circles) in blood over 10 h following a single iv dose of 100 mg/kg of 14C-labeled methanol in male Fischer-344 rats. Each point represents mean value of experimental data (n = 4). View larger version (15K): [in this window] [in a new window] FIG. 3. (A) Model simulations (lines) compared with experimental data of Horton groups.yahoo.com/group/aspartameNM/messages
Report this post as:
also aspartame (methanol, formaldehyde, formic acid) [ Continued ]by Rich Murray Monday, Jan. 03, 2005 at 12:25 PMrmforall@comcast.net 505-986-9103 1943 Otowi Road, Santa Fe, New Mexico 87505 [ Continued ]
FIG. 3. (A) Model simulations (lines) compared with experimental data of Horton et al. (1992) on the cumulative urinary excretion profiles of methanol (crossbars) and formate (circles) over 18 h following a single iv dose of 100 mg/kg of 14C-labeled methanol in male Fischer-344 rats. Each point represents mean value of experimental data (n = 4). (B) Model simulations (lines) compared with experimental data of Horton et al. (1992) on the cumulative exhalation profiles of methanol (crossbars) and CO2 (squares) over 18 h following a single iv dose of 100 mg/kg of 14C-labeled methanol in male Fischer-344 rats. Each point represents mean value of experimental data (n = 4). The estimated average Michaelis-Menten affinity constant value reported in Table 2 and determined using the iv data (Km-IV of 770 µmol, which represents the body burden of methanol corresponding to half of the maximal velocity for methanol metabolism) shows that when injecting 100 mg/kg of 14C-methanol to Fischer rats (700 µmol), metabolism is not yet saturated. From the product of Km-IV and kmet, an average Vmax value of 411 µmol/h can be calculated. The model predicts that methanol elimination from the whole body is quite rapid (mean elimination half-life of 1.3 h) and that, on average, only 0.01% of methanol remains in the unchanged form 18 h following iv injection of 100 mg/kg of 14C-methanol in rats. Peak levels of free formaldehyde in the whole body are reached 0.5 h postdosing, at which time formaldehyde burden represents on average 3.2% of the injected dose. Virtually no free formaldehyde remains in the body 18 h postexposure. The metabolism of methanol to formaldehyde (kmet) is predicted to be the rate limiting step in the whole body elimination kinetics of free formaldehyde. Indeed, the biotransformation of formaldehyde to its by-products is estimated to be very rapid (kform + koth being very large) compared to methanol metabolism to formaldehyde (kmet), as apparent when comparing reports of McMartin et al. (1979) and Horton et al. (1992). On the other hand, according to model predictions, peak levels of unbound formate in the whole body are reached only 3-3.5 h postexposure where average formate burden represents 20.1% of injected 14C-methanol. Eighteen h postexposure, on average 0.5% of the dose remains in the body as free formate. Initial build-up of unbound formate in the body prior to attrition is dependent on the fact that the metabolism rate constant of formaldehyde to formate (kform) is very rapid (average half-life of about 10 min) compared to the major elimination route of formate, the metabolism rate to CO2 and subsequent exhalation (kCO2), for which a mean half-life of 2.2 h can be calculated. Since the urinary excretion of formate is negligible compared to CO2 exhalation, the former contributes only marginally to the whole body time course of formate. In fact, the model predicts that on average 48.8% of the 14C-methanol iv dose is eliminated as exhaled CO2 as compared to 1.7% as urinary formate, which is congruent with the experimental results of Horton et al. (1992). In comparison, it is estimated from the model that on average 0.8% of the dose is excreted as unchanged methanol in the urine and 2.4% of body methanol is exhaled unchanged again in accordance with the experimental data of Horton et al. (1992). Model Validation Using the Inhalation Data of Horton et al. (1992) in Fischer-344 Rats With the parameter values determined using the iv data of Horton et al. (1992), the model was applied to another set of data from the same authors on the blood concentration-time profiles of methanol during and following 6-h inhalation exposures to 200, 1200, and 2000 ppm of methanol in male Fischer-344 rats. It gave a good prediction of the time-course curves for the 2 lowest doses but underestimated the blood concentrations for the 2000 ppm dose (data not shown). Thus, a new value of the saturation constant Km was estimated from a statistical best-fit on the blood concentration-time profile data of Horton et al. (1992) in male Fischer-344 rats exposed by inhalation to 2000 ppm of methanol vapors for 6 h (Km-Inh) (see Table 2). This Km-Inh value was about 3 times smaller than that determined with the iv data (on average 235 µmol). Thus, after inhalation exposure to 2000 ppm, saturation of methanol metabolism appears to occur at a lower body burden. With this Km-Inh value, a Vmax of 125 µmol/h was calculated. Using this newly determined Km constant for an inhalation exposure, the proposed model provided a close approximation to the data of Horton et al. (1992) on the blood concentration-time profiles of methanol in male Fischer-344 rats exposed to vapor concentrations of 200, 1200, and 2000 ppm of methanol for 6 h (Fig. 4). View larger version (18K): [in this window] [in a new window] FIG. 4. Model simulations (lines) compared with experimental data of Horton et al. (1992) on the time courses of methanol concentrations in blood during and following 6-h inhalation exposures to 200 (diamonds), 1200 (crossbars), and 2000 (squares) ppm of methanol vapors in male Fischer-344 rats. Each point represents mean value of experimental data (n = 4). Model Adapted to Female Cynomolgus Monkey Data of Dorman et al. (1994) Using the conceptual and functional representation of the model established with rat data, the model was adapted to monkeys by adjusting parameters values (see Table 2), through a statistical best-fit, to the data of Dorman et al. (1994) in female cynomolgus monkeys exposed by inhalation to methanol vapors. As observed in rats, pulmonary absorption of methanol was estimated to be very rapid (a few minutes) as compared to the metabolism rate constant kmet of whole body methanol to formaldehyde. The predicted pulmonary absorption fraction of methanol in monkeys was in the same range as that determined in rats. The estimated monkey constant kmet was however 1.8 times higher than in rats. Interestingly, contrary to the rat, according to the data of Dorman et al. (1994), no saturation of methanol metabolism was apparent in monkeys even after a 2-h inhalation exposure to 2000 ppm. Further comparison of monkey and rat parameter values shows that the estimated monkey metabolism rate constant kform of whole body formaldehyde to formate was 2.0 times lower than that of rats. This was also the case for exhalation rate constant kex of absorbed methanol (1.8 times). The monkey kCO2 value, which represents a combined metabolism rate constant of whole body formate to CO2 and transfer rate constant of CO2 to the exhaled air, was estimated to be 2.6 times higher than in rats. As observed with the rat data of Horton et al. (1992), no saturation of formaldehyde or formate metabolism was apparent from the data of Dorman et al. (1994). It is also noteworthy that the estimated monkey transfer rate constant ku of whole body formate to urine was 5.4 times lower than in rats and the monkey transfer rate constant km of whole body methanol to urine was 12.8 times smaller than that obtained for rats. The estimated monkey apparent volume of distribution of methanol and formate, expressed in liters per kilogram of body weight, were only slightly lower than those of the rats (1.2 and 1.4 times, respectively). With the parameter values described in Table 2, Figure 5 shows that the model provides a close approximation to the data obtained by Dorman et al. (1994) on the blood concentration-time profiles of methanol and formate as well as the time dependent variations in methanol and CO2 exhalation rates over the 8-h period following the beginning of a 2-h inhalation exposure to 900 ppm of 14C-methanol in adult female cynomolgus monkeys. Although the corresponding detailed urinary excretion profiles of methanol and formate were not depicted by Dorman et al. (1994), cumulative excretion of methanol and formate in urine was reported. The model succeeded in reproducing closely these values (0.43 µmol predicted as compared to 0.41 µmol observed on average for urinary methanol, and 1.12 µmol predicted as compared to 1.15 µmol observed on average for urinary formate). View larger version (16K): [in this window] [in a new window] FIG. 5. (A) Model simulations (lines) compared with experimental data of Dorman et al. (1994) on the time courses of methanol (crossbars) and formate (circles) concentrations in blood during and following a 2-h inhalation exposure to 900 ppm of 14C-methanol in adult female cynomolgus monkeys (Macaca fascicularis). Each point represents mean value of experimental data (n = 4). (B) Model simulations (lines) compared with experimental data of Dorman et al. (1994) on the time courses of methanol (crossbars) and CO2 (squares) exhalation rates during and following a 2-h inhalation exposure to 900 ppm of 14C-methanol in adult female cynomolgus monkeys. Each point represents mean value of experimental data (n = 4). Model Validation Using the Inhalation Data of Male Rhesus Monkeys of Horton et al. (1992) The model, with parameter values adapted as above to cynomolgus monkey data of Dorman et al. (1994), was further validated using experimental results of Horton et al. (1992) in young male rhesus monkeys exposed for 6 h to vapor concentrations of 1200 and 2000 ppm (These authors also exposed monkeys to 200 ppm but concentration values were too small to provide an accurate prediction.) It was assumed that differences in the kinetics were mainly a result of interstrain differences in the metabolism rate of methanol, kmet. With a smaller average kmet value of 0.22/h determined by statistical best-fit for larger male rhesus monkeys (5-7 kg) as compared to 0.96/h for smaller female cynomolgus monkeys (3.5-5 kg), the model was able to reproduce the concentration-time course data of methanol in blood of Horton et al. (1992) as seen in Figure 6. View larger version (14K): [in this window] [in a new window] FIG. 6. Model simulations (lines) compared with experimental data of Horton et al. (1992) on the time courses of methanol concentrations in blood following 6-h inhalation exposures to 1200 (crossbars) and 2000 (circles) ppm of methanol vapors in young male rhesus monkeys (Macaca mulatta). Each point represents mean value of experimental data (n = 3). It is also interesting to note that, as reported by Horton et al. (1992), the model predicts that blood formate concentrations in monkeys will not exceed endogenous background values even for 6-h inhalation exposures to 2000 ppm of methanol vapors (data not shown). This was also observed when simulating the data of Dorman et al. (1994) on the blood concentration-time course curve of formate in monkeys exposed to 900 ppm of methanol vapors for 2 h. Model Adapted to the Human Data of Osterloh et al. (1996) and Sedivec et al. (1981) The parameter values estimated by fitting the model to the observed data of Osterloh et al. (1996) and Sedivec et al. (1981) on the disposition of methanol and its metabolites in humans are presented in Table 2. The estimated value of kmet was in the same range as that determined in animals. The km value was estimated to be close to that obtained in rats (1.5 times lower) but 8.3 times higher than that of monkeys. Figure 7 shows that the model simulates correctly the data obtained by Osterloh et al. (1996) on the concentration-time course of blood methanol in human volunteers exposed by inhalation to 200 ppm of methanol for 4 h. The model included a constant background whole body methanol burden of 2133 µmol, which corresponds to the mean blood concentration of 1.5 mg/l of methanol measured by Osterloh et al. (1996) in control subjects at the end of an 8-h frequent blood sampling period. In accordance with the experimental data of Osterloh et al. (1996), the model predicts a log-linear elimination of blood methanol over the 4-h sampling period following exposure (data not shown), indicating the absence of saturation of methanol metabolism for the 4-h inhalation exposure at 200 ppm. View larger version (15K): [in this window] [in a new window] FIG. 7. Model simulations (solid lines) compared with experimental data of Osterloh et al. (1996) on the blood concentration-time profile of methanol in human volunteers during and following a 4-h inhalation exposure to 200 ppm of methanol vapors (crossbars). Background blood methanol concentration values considered for model simulations (dashed lines) and experimentally determined by Osterloh et al. (1996) over the course of their experimental study (circles) are also represented. Symbols represent mean experimental values from 22 subjects. Figure 8 compares model simulations to the time dependent cumulative excretion of methanol in human volunteers during and following 8-h inhalation exposures to 102, 205, and 300 mg/m3, as determined from the data of Sedivec et al. (1981). A mean background whole body burden of methanol of 700 to 1000 µmol depending on the dose (equivalent to a blood concentration of 0.4-0.6 mg/l) was included in the model, which gives predicted baseline urinary concentrations in the same value range as those experimentally observed (0.7 mg/l on average). With these initial conditions, predictions were in close agreement with the experimentally observed data. View larger version (17K): [in this window] [in a new window] FIG. 8. Model simulations (lines) compared with experimental data of Sedivec et al. (1981) on the cumulative urinary excretion time-course of methanol in human volunteers during and following 8-h inhalation exposures to 102 (diamonds), 205 (crossbars), and 300 (squares) mg/m3 of methanol. Symbols represent mean experimental values from 4 subjects. Model Validation Using the Inhalation Data of Batterman et al. (1998) in Human Volunteers The model adapted to humans was applied to the data of Batterman et al. (1998) in human volunteers exposed to methanol vapor concentrations of 800 ppm for 30 min, 1 h, and 2 h. All the parameter values were kept as determined with the previous human data of Osterloh et al. (1996) and Sedivec et al. (1981) except for the pulmonary retention. Indeed, for a proper simulation of the experimental data, the average pulmonary absorption fraction had to be 0.76, 0.82, and 0.81 for the 30 min, 1, and 2 h exposures, respectively, which is congruent with the mean value of 0.79 reported by Batterman et al. (1998). With these values, Figures 9-11 show that simulations were in close agreement with the experimentally determined concentration-time profiles of methanol in blood, as well as the time evolution of methanol cumulative excretion in urine and in the exhaled air for the various exposure scenarios. A good fit was obtained even when neglecting background methanol in the whole body. Regarding Figure 11, the exhalation rate is almost constant during the exposure, which explains the plateau for the cumulative exhalation. View larger version (16K): [in this window] [in a new window] FIG. 9. Comparison of model simulations (lines) with experimental data (symbols represent mean values from 4 subjects) of Batterman et al. (1998) on the blood concentration-time profiles of methanol in 3 groups of human volunteers during and following inhalation exposures to 800 ppm of methanol vapors for 30 min (A), 1 h (B), and 2 h (C). View larger version (14K): [in this window] [in a new window] FIG. 10. Comparison of model simulations (lines) with experimental data (symbols represent mean values from 4 subjects) of Batterman et al. (1998) on the cumulative urinary excretion time courses of methanol in 3 groups of human volunteers during and following inhalation exposures to 800 ppm of methanol vapors for 30 min (A), 1 h (B), and 2 h (C). View larger version (15K): [in this window] [in a new window] FIG. 11. Comparison of model simulations (lines) with experimental data (symbols represent mean values from 4 subjects) of Batterman et al. (1998) on the cumulative exhalation time courses of methanol in 3 groups of human volunteers during and following inhalation exposures to 800 ppm of methanol vapors for 30 min (A), 1 h (B), and 2 h (C). Prediction of the Time Course Curves of Methanol and Formate Concentrations in Blood and Urine during a 5-Day Continuous Exposure to Methanol Vapor Concentrations of 200 ppm in Humans The model can also be used to predict the time-dependent variations of methanol and formate concentrations in human blood and urine during a continuous inhalation exposure to 200 ppm of methanol, considering a negligible background burden of methanol, an absorption fraction of 0.577, a pulmonary ventilation rate of 10.8 l/min, and a daily urinary excretion rate of 1.5 l. Near steady state levels were reached within 20 h following the start of exposure. At the end of a 5-day exposure period, predicted blood concentration of methanol was 5.5 mg/l (171 µmol/l) and that of formate was 0.16 mg/l (3.5 µmol/l). The latter formate concentration was obtained by considering an apparent volume of distribution of formate in humans (in l/kg of body weight) similar to that calculated for monkeys. With this exposure level, the model predicts near steady state urinary concentrations of methanol of 8.1 mg/l (252 µmol/l) and of formic acid of 1.5 mg/l (31.7 µmol/l, 0.97 mg/g creatinine, or 2390 µmol/mol creatinine). Thus, at the end of a 5-day continuous inhalation exposure to 200 ppm of methanol vapors, predicted methanol concentrations in blood and urine were 5 to 11 times greater than reported mean background values of unexposed subjects (1 mg/l in blood and 0.73 mg/l in urine) (Osterloh et al., 1996; Sedivec et al., 1981). On the other hand, predicted concentrations of blood formate and urinary formic acid in humans (0.16 and 1.5 mg/l, respectively), although in accordance with the experimental data from methanol exposures in primates and humans, were well below mean background values of unexposed subjects (4.9-10.3 mg/l in blood and 6.3-13 mg/l in urine) reported by various authors (Baumann and Angerer, 1979; D'Alessandro et al., 1994; Heinrich and Angerer, 1982; Lee et al., 1992; Osterloh et al., 1996). The model simulations suggest that an 8-h inhalation exposure of at least 500 to 2000 ppm, without physical activities, would be necessary for blood formate and urinary formic acid concentrations to reach reported mean background values. The exact exposure levels necessary depend on the values assumed for the absorption fraction, the pulmonary ventilation rate, and the daily urinary excretion rate. There are considerable variations in the literature for these parameters. Model Description of the Kinetics of Methanol and Its Metabolites A biologically based dynamic model was developed to simulate the uptake and disposition of methanol and its metabolites (formaldehyde, formate, CO2) in animals and humans. Based on the in vivo time profiles of methanol, formate, and CO2 in blood and accessible biological matrices, the model was able to reproduce the essential kinetic processes of methanol disposition. It can now be used to quantitatively relate the parent compound or the metabolites in biological matrices to the absorbed dose and tissue burdens at any point in time in rats, monkeys, and humans for different exposure situations, thus reducing the uncertainties in the dose-response relationship, animal-to-human, and exposure scenario comparisons. The model showed that the kinetics of systemic methanol were dependent on the pulmonary uptake and on the metabolism of methanol to formaldehyde. The pulmonary absorption fraction and ventilation rate were the only model parameters that needed to be modified within a species to provide a good prediction of all the data sets. These predicted parameters were, however, in the value range reported in the published literature (Dorman et al., 1994; Fisher et al., 2000; Horton et al., 1992; Sedivec et al., 1981). The pulmonary absorption fraction did not appear to be influenced by the exposure level or duration nor by the pulmonary ventilation rate, as observed previously by Sedivec et al. (1981) and Medinsky et al. (1997). For all the other parameters, a single set of values for a given species and strain was found to provide a close approximation to the available kinetic time profile data. In particular, a single average value for the metabolism rate constants can be used in the model for a given exposure route. In accordance with the published animal data (Dorman et al., 1994; Horton et al., 1992), the model predicts that absorbed methanol is eliminated mainly by metabolism to formaldehyde and that only a minor fraction of the exposure dose is eliminated as unchanged methanol in urine. On the other hand, from the rat data of Horton et al. (1992), differences in the saturation of methanol metabolism were observed depending on the exposure route. Indeed, in the rat model, a same value for the metabolism rate constant of whole body methanol to formaldehyde, kmet, was found to provide a close approximation to the data of Horton et al. (1992) on the blood concentration-time profiles of methanol after iv and inhalation exposures to methanol in Fischer-344 rats. However, the Km value determined using the inhalation data was 3 times smaller than that obtained using the iv data. Although methanol has been reported to be metabolized mainly in the liver, pulmonary metabolism is also likely to occur. Indeed, the catalase-peroxidase system responsible for a major fraction of methanol metabolism in rats is widely distributed in mammalian tissues (Housset, 1986; Morikawa and Harada, 1969; Sugata et al., 1979). It is, in particular, present in the membranes of the upper respiratory tract, the main site of pulmonary absorption of methanol (Perkins et al., 1996). Of course, given that the Km parameters were estimated from mean blood concentration data without taking into account interindividual variations, it cannot be excluded that there is no significant difference between the 2 route-specific Km values. It should be remembered that, only in the case of methanol metabolism to formaldehyde was a saturation constant necessary. As mentioned previously, for the exposure dose range of the studies on which the model is based, no saturation of formate or CO2 metabolism was apparent (Dorman et al., 1994; Horton et al., 1992; Osterloh et al., 1996; Sedivec et al., 1981). Though the saturation of formate metabolism has been reported after very high iv doses of sodium formate in rats (164, 328, and 492 mg/kg; Damian and Raabe, 1996) and appeared to occur in a case of methanol poisoning in a human subject (Jacobsen et al., 1988), under inhalation exposures levels in occupational and general environments, it is unlikely to occur. According to model predictions, congruent with the data in the literature (Dorman et al., 1994; Horton et al., 1992), a certain fraction of formaldehyde is readily oxidized to formate, a major fraction of which is rapidly converted to CO2 and exhaled, whereas a small fraction is excreted as formic acid in urine. However, fits to the available data in rats and monkeys of Horton et al. (1992) and Dorman et al. (1994) show that, once formed, a substantial fraction of formaldehyde is converted to unobserved forms. This pathway contributes to a long-term unobserved compartment. The latter, most plausibly, represents either the formaldehyde that (directly or after oxidation to formate) binds to various endogenous molecules (Heck et al., 1983; Røe, 1982) or is incorporated in the tetrahydrofolic-acid-dependent one-carbon pathway to become the building block of a number of synthetic pathways (Røe, 1982; Tephly and McMartin, 1984). That substantial amounts of methanol metabolites or by-products are retained for a long time is verified by Horton et al. (1992) who estimated that 18 h following an iv injection of 100 mg/kg of 14C-methanol in male Fischer-344 rats, only 57% of the dose was eliminated from the body. From the data of Dorman et al. (1994) and Medinsky et al. (1997), it can further be calculated that 48 h following the start of a 2-h inhalation exposure to 900 ppm of 14C-methanol vapors in female cynomolgus monkeys, only 23% of the absorbed 14C-methanol was eliminated from the body. These findings are corroborated by the data of Heck et al. (1983) showing that 40% of a 14C-formaldehyde inhalation dose remained in the body 70 h postexposure. In the present study, the model proposed rests on acute exposure data, where the time profiles of methanol and its metabolites were determined only over short time periods (a maximum of 6 h of exposure and a maximum of 48 h postexposure). This does not allow observation of the slow release from the long-term components. It is to be noted that most of the published studies on the detailed disposition kinetics of methanol regard controlled short-term (iv injection or continuous inhalation exposure over a few hours) methanol exposures in rats, primates, and humans (Batterman et al., 1998; Damian and Raabe, 1996; Dorman et al., 1994; Ferry et al., 1980; Fisher et al., 2000; Franzblau et al., 1995; Horton et al., 1992; Jacobsen et al., 1988; Osterloh et al., 1996; Pollack et al., 1993; Sedivec et al., 1981; Ward et al., 1995; Ward and Pollack, 1996). Experimental studies on the detailed time profiles following controlled repeated exposures to methanol are lacking. Data on methanol and formate concentrations in spot blood and urine samples of chronically exposed workers (Baumann and Angerer, 1979; Kawai et al., 1991; Yasugi et al., 1992) are available but uncertainties regarding the exposure dose and concomitant exposure to other chemicals limit their use in the elaboration of a kinetic model. With regard to the apparent volume of distribution of methanol, which was calculated in the current study using classic approaches (see Method and Model Presentation), it was expected that its value would correspond approximately to the whole body water content. The slightly larger weight adjusted volume of distribution of methanol calculated in rats (0.92 l/kg of body weight) as compared to monkeys (0.77 l/kg of body weight) can be explained by the smaller adipose tissue fraction of body weight in rats. As for the apparent volume of distribution of formate determined in this study, the weight adjusted values calculated in rats and monkeys were in the same range, although slightly higher in rats than in monkeys (6.4 and 4.6 l/kg of body weight, respectively). However, the volume of distribution of formate was larger than that of methanol, which strongly suggests that formate distributes in body constituents other than water, such as proteins. The closeness of our simulations to the available experimental data on the time course of formate blood concentrations is consistent with the volume of distribution concept (i.e., rapid exchanges between the nonblood pool of formate and blood formate). Species Differences in the Kinetics of Methanol and Its Metabolites Critical biological determinants of species differences in the disposition of methanol and its metabolites were determined from in vivo data from several studies (Batterman et al., 1998; Fisher et al., 2000; Horton et al., 1992; Sedivec et al., 1981). In agreement with their findings, the model predicts that the average pulmonary absorption fraction fabs of methanol and the metabolism rate constant kmet of whole body methanol to formaldehyde were in the same value range in rats, monkeys, and humans (on average 0.58-0.82 for fabs and 0.219-0.96/h for kmet). However, the saturation of methanol metabolism appeared to occur at a lower exposure dose in rats than in monkeys and humans. Indeed, from the data of Horton et al. (1992) on the blood concentration-time profile of methanol in rats exposed to 2000 ppm of methanol vapors for 6 h, a Km value of 36.6 mg/l of blood and Vmax of 19.4 mg/l/h were estimated in the current study whereas following a similar exposure in monkeys, no saturation of methanol metabolism was apparent. The model also predicts that there is no saturation of methanol metabolism from the data of Batterman et al. (1998) in human volunteers exposed to 800 ppm of methanol vapors for 2 h nor from those of Sedivec et al. (1981) in volunteers exposed to 229 ppm of methanol vapors for 8 h. Interestingly, a striking species difference in the kinetics was attributed to a metabolism rate constant ratio kform/kfald of whole body formaldehyde to formate twice as high in rats than in monkeys (0.53 vs. 0.26). Thus, in monkeys and plausibly humans, a much larger fraction of body formaldehyde is rapidly converted to unobserved forms rather than passed on to formate and eventually CO2. Comparison of the Current Model with Others Previously Published The current biologically based dynamic model can be compared to some of the previously published models. In particular, Horton et al. (1992) developed a PBPK model to describe the kinetics of methanol and its metabolites in rats, monkeys, and humans. Their model was comprised of 4 compartments: liver, kidney, and richly and slowly perfused tissues. As in our model, the metabolism of methanol to formaldehyde was assumed to be the main biological determinant of methanol elimination kinetics. In addition, in both the current model and that published by Horton et al. (1992), not only were the kinetics of methanol in blood, urine, and exhaled air modeled but also the time evolution of formate in blood and urine and of CO2 in the exhaled air. However, in the study of Horton et al. (1992), 2 saturable metabolic pathways for methanol metabolism were considered whereas in our study, even by introducing only 1 metabolism route for methanol, with saturable elimination, the model gave a good prediction of the experimental data. In the PBPK model of Horton et al. (1992), the metabolism of formate and CO2 was also assumed to follow Michaelis Menten kinetics. In our model, as mentioned previously, no saturation constants for these metabolism processes were introduced since fits to the available time course data suggested the absence of saturation of formate and CO2 metabolism in the exposure dose range used in the studies on which the model is based. Furthermore, conceptual and functional differences between the current model and the PBPK model of Horton et al. (1992) are related to the fact that the current model compartmentalization is dependent on the availability of experimental data on the detailed time course of methanol and its metabolites in blood, tissues, and excreta and on the hierarchy of the time scales for the various biological processes. The main structural difference between our model and that of Horton et al. (1992) concerns our regrouping into a single compartment the methanol body burden whereas Horton et al. (1992) have fragmented the body into several compartments according to the general PBPK structure. In our model, methanol body burden regrouping relies on the fact that methanol distributes uniformly and rapidly in total body water and thus the apparent volume of distribution of methanol corresponds to the total body water content. This allowed us to reduce the number of parameters to be determined to describe the overall model dynamics of methanol. Based on the available data on methanol blood kinetics for the 3 species studied, this regrouping also enabled the determination of species specific parameters by direct fits, without the need for allometric extrapolation. Furthermore, the current model ensures conservation of mass by the introduction of an unobserved metabolite compartment. In the model of Horton et al. (1992), to account for the fraction of the methanol dose that was unobserved experimentally and thus to obtain a good fit to their experimental data on the cumulative exhalation of CO2 in rats exposed to 14C-labeled methanol, the rate of formate metabolism had to be multiplied by 0.6 to correspond to the fraction of the methanol dose eventually excreted as CO2 over the 18-h sample collection period of their study. More recently, Fisher et al. (2000) published a PBPK model for monkeys to describe the kinetics of methanol. The structure of their PBPK model for methanol was similar to that of Horton et al. (1992) but also accounted for the fractional systemic uptake of inhaled methanol vapors in the lungs. This fractional systemic uptake was also introduced in our model. Prediction of the Most Useful Biological Indicator of Exposure to Methanol The biological monitoring of exposure, through the analysis of blood concentrations or urinary and exhaled levels, has become an increasingly popular means of estimating the absorbed dose. This model can be used in conjunction with biological measurements of methanol and formate or formic acid to determine the level of exposure and subsequent build-up in tissues. It can also help to establish the best biomarker of exposure, the sampling strategy for routine monitoring and the significance of measurements at different times. Since systemic formate is thought to be responsible for a large part of the deleterious effects induced by methanol exposures (Tephly and McMartin, 1984), the measurement of blood formate or urinary formic acid appears interesting a priori for the biological monitoring of exposure to methanol. However, the model shows that background concentrations of formate are much higher than those stemming from fairly high methanol exposures. Indeed, the model, adapted to kinetic data in human volunteers exposed acutely to methanol vapors, predicts that 8-h inhalation exposures ranging from 500 to 2000 ppm are needed to increase blood formate concentrations above reported mean endogenous values of 4.9 to 10.3 mg/l (Baumann and Angerer, 1979; Lee et al., 1992; Osterloh et al., 1996), and for urinary formic acid concentrations to reach the published mean background values of 6.3 to 13 mg/l (Baumann and Angerer, 1979; D'Alessandro et al., 1994; Heinrich and Angerer, 1982). The monkey data of Dorman et al. (1994) show that even after a 2-h inhalation exposure to 900 ppm of 14C-methanol in female cynomolgus monkeys, 14C-formate concentrations in blood were far below normal endogenous values. Likewise, studies in human volunteers acutely exposed to methanol, at the level of 200 ppm, concur to indicate that blood formate and urinary formic acid concentrations remain within the background value range of unexposed subjects (D'Alessandro et al., 1994; Franzblau et al., 1993; Lee et al., 1992; Osterloh et al., 1996). Only in the studies of Kawai et al. (1991) and Yasugi et al. (1992) was a significant correlation between the urinary excretion of formic acid and exposure to methanol vapors observed. However, the workers were exposed to airborne concentrations of methanol of up to 4000 ppm over an 8-h workshift. These findings suggest that it is not justified to monitor concentrations of blood formate or urinary formic acid at methanol exposure levels in the range of or below the airborne threshold limit value of 200 ppm for occupational settings. If toxic effects do occur following low level methanol exposures, the mode of action is not likely to be through the accumulation of formate. As suggested by some reports (Cook et al., 1991; Kingsley and Hirsch, 1954), it may rather be attributable to methanol itself. The use of formate as a biomarker of exposure to methanol is further limited by the fact that it is not a specific metabolite of methanol exposure. Also, background concentrations of formate are subject to wide interindividual variations (Baumann and Angerer, 1979; D'Alessandro et al., 1994; Franzblau et al., 1995; Heinrich and Angerer, 1982; Lee et al., 1992; Osterloh et al., 1996; Sedivec et al., 1981). This leaves blood and urinary methanol as the most appropriate biomarkers of absorbed methanol. Since the model relates blood and urinary methanol burdens to the exposure dose and body burdens of metabolites at all time points, it can be of great use in reconstructing past and present exposure levels starting from methanol amounts in blood and urine. Methanol Kinetics: First Order Linear Differential Equations for Each Compartment Kinetics of the Methanol Form From Figure 1, the following differential equations are obtained (see Table 1 for definitions of symbols): ((1)) where g(t) is the pulmonary exposure dose per unit of time. g(t) = Cexp x VR where Cexp is the exposure concentration and VR is the pulmonary ventilation rate. For an iv injection, g(t) = 0 for t > 0 and at time t = 0, X(0) = 100% of dose. The fraction of absorption through the lungs can be defined as fabs = kabs/(kabs + kre). ((2)) where Considering the rapid exchange rates between the various internal organs and blood, and thus between the whole body burden and blood, it can be considered that ((3)) where B(t) is the blood concentration of methanol as a function of time. ((4)) ((5)) Kinetics of the Formaldehyde Form ((6)) The global breakdown rate of formaldehyde, kfald = kform + koth, is very large compared to the subsequent transfer rates {ku and kCO2} because observed formaldehyde levels are very small compared to formate levels. This implies rapid breakdown of formaldehyde compared to formate elimination rates. The rate of formaldehyde breakdown, kfald, was given the value reported by McMartin et al. (1979) in cynomolgus monkeys, corresponding to a half-life of 1.5 min. However, its exact value is not relevant to the model's unfolding, only the ratios kform/kfald and koth/kfald are. Kinetics of the Other Forms ((7)) ((8)) ((9)) Kinetics of the Formate Form ((10)) ((11)) ((12)) Mass Balance Verification Batterman, S. A., Franzblau, A., D'Arcy, J. B., Sargent, N. E., Gross, K. B., and Schreck, R. M. (1998). Breath, urine, and blood measurements as biological exposure indices of short-term inhalation exposure to methanol. Int. Arch. Occup. Environ. Health 71, 325-335.[Medline] Baumann, K., and Angerer, J. (1979). Occupational chronic exposure to organic solvents. VI. Formic acid concentration in blood and urine as an indicator of methanol exposure. Int. Arch. Occup. Environ. Health 42, 241-249.[Medline] Bolon, B., Dorman, D. C., Janszen, D., Morgan, K. T., and Welsch, F. (1993). Phase-specific developmental toxicity in mice following maternal methanol inhalation. Fundam. Appl. Toxicol. 21, 508-516.[Medline] Carrier, G., Bouchard, M., Brunet, R. C., and Caza, M. (2001a). A toxicokinetic model for predicting the tissue distribution and elimination of organic and inorganic mercury following exposure to methyl mercury in animals and humans. II. Application and validation of the model in humans. Toxicol. Appl. Pharmacol. 171, 50-60.[Medline] Carrier, G., and Brunet, R. C. (1999). A toxicokinetic model to assess the risk of azinphosmethyl exposure in humans through measures of urinary elimination of alkylphosphates. Toxicol. Sci. 47, 23-32.[Abstract] Carrier, G., Brunet, R. C., and Brodeur, J. (1995a). Modeling of the toxicokinetics of polychlorinated dibenzo-p-dioxins and dibenzofurans in mammalians, including humans. I. Nonlinear distribution of PCDD/PCDF body burden between liver and adipose tissues. Toxicol. Appl. Pharmacol. 131, 253-266.[Medline] Carrier, G., Brunet, R. C., and Brodeur, J. (1995b). Modeling of the toxicokinetics of polychlorinated dibenzo-p-dioxins and dibenzofurans in mammalians, including humans. II. Kinetics of absorption and disposition of PCDDs/PCDFs. Toxicol. Appl. Pharmacol. 131, 267-276.[Medline] Carrier, G., Brunet, R. C., Caza, M., and Bouchard, M. (2001b). A toxicokinetic model for predicting the tissue distribution and elimination of organic and inorganic mercury following exposure to methyl mercury in animals and humans. I. Development and validation of the model using experimental data in rats. Toxicol. Appl. Pharmacol. 171, 38-49.[Medline] Cook, M. R., Bergman, F. J., Cohen, H. D., Gerkovich, M. M., Graham, C., Harris, R. K., and Siemann, L. G. (1991). Effects of Methanol Vapor on Human Neurobehavioral Measures. Research Report Number 42. Health Effects Institute, Cambridge, MA. D'Alessandro, A., Osterloh, J. D., Chuwers, P., Quinlan, P. J., Kelly, T. J., and Becker, C. E. (1994). Formate in serum and urine after controlled methanol exposure at the threshold limit value. Environ. Health Perspect. 102, 178-181.[Medline] Damian, P., and Raabe, O. G. (1996). Toxicokinetic modeling of dose-dependent formate elimination in rats: In vivo-in vitro correlations using the perfused rat liver. Toxicol. Appl. Pharmacol. 139, 22-32.[Medline] Dorman, D. C., Moss, O. R., Farris, G. M., Janszen, D., Bond, J. A., and Medinsky, M. A. (1994). Pharmacokinetics of inhaled [14C]methanol and methanol-derived [14C]formate in normal and folate-deficient cynomolgus monkeys. Toxicol. Appl. Pharmacol. 128, 229-238.[Medline] Downie, A., Khattab, T. M., Malik, M. I. A., and Samara, I. N. (1992). A case of percutaneous industrial methanol toxicity. Occup. Med. 42, 47-49.[Abstract] Ferry, D. G., Temple, W. A., and McQueen, E. G. (1980). Methanol monitoring. Comparison of urinary methanol concentration with formic acid excretion rate as a measure of occupational exposure. Int. Arch. Occup. Environ. Health 47, 155-163.[Medline] Fisher, J. W., Dorman, D. C., Medinsky, M. A., Welsch, F., and Conolly, R. B. (2000). Analysis of respiratory exchange of methanol in the lung of the monkey using a physiological model. Toxicol. Sci. 53, 185-193.[Abstract/Free Full Text] Franzblau, A., Batterman, S. A., D'Arcy, J. B., Sargent, N. E., Gross, K. B., and Schreck, R. M. (1995). Breath monitoring of inhalation and dermal methanol exposure. Appl. Occup. Environ. Hyg. 10, 833-839. Franzblau, A., Lee, E. W., Schreck, R. M., D'Arcy, J. B., Santrock, J., and Levine, S. P. (1993). Absence of formic acid accumulation in urine following five days of methanol exposure. Appl. Occup. Environ. Hyg. 8, 883-888. Goodman, J. I., and Tephly, T. R. (1968). The role of hepatic microbody and soluble oxidases in the peroxidation of methanol in the rat and monkey. Mol. Pharmacol. 4, 492-501.[Abstract] Health Effects Institute (1987). Automotive Methanol Vapors and Human Health: An Evaluation of Existing Scientific Information and Issues for Future Research (a Special Report). Health Effects Institute, Cambridge, MA. Heck, H. A., Chin, T. Y., and Schmitz, M. C. (1983). Distribution of [14C] formaldehyde in rats after inhalation exposure. In Formaldehyde Toxicity (J. E. Gibson, Ed.), pp. 26-37. Hemisphere Publishing Corporation, Washington, DC. Heinrich, R., and Angerer, J. (1982). Occupational chronic exposure to organic solvents. X. Biological monitoring parameters for methanol exposure. Int. Arch. Occup. Environ. Health 50, 341-349.[Medline] Horton, V. L., Higuchi, M. A., and Rickert, D. E. (1992). Physiologically based pharmacokinetic model for methanol in rats, monkeys, and humans. Toxicol. Appl. Pharmacol. 117, 26-36.[Medline] Housset, B. (1986). Mechanisms of oxygen-induced lung injury. Arch. Int. Physiol. Biochim. 94, S1-5. IPCS (1997). Environmental Health Criteria 196-Methanol. International Programme on Chemical Safety, World Health Organization, Geneva. Jacobsen, D., Webb, R., Collins, T. D., and McMartin K. E. (1988). Methanol and formate kinetics in late diagnosed methanol intoxication. Med. Toxicol. Adverse Drug Exp. 3, 418-423.[Medline] Kavet, S., and Nauss, K. M. (1990). The toxicity of inhaled methanol vapors. Crit. Rev. Toxicol. 21, 21-50.[Medline] Kawai, T., Yasugi, T., Mizunuma, K., Horiguchi, S., Hirase, Y., Uchida, Y., and Ikeda, M. (1991). Methanol in urine as a biological indicator of occupational exposure to methanol vapor. Int. Arch. Occup. Environ. Health 63, 311-318.[Medline] Kingsley, W. H., and Hirsch, F. G. (1954). Toxicologic considerations in direct process spirit duplicating machines. Comp. Med. 6, 7-8. Knuiman, J. T., Hautvast, A. J., van der Heyden, L., Geboers, J., Joossens, J. V., Tornquist, H., Isaksson, B., Pietinen, P., Tuomilehto, U., Poulsen, L., Flynn, A., Shortt, C., Böing, H., Yomtov, B., Magalhaes, E., Angelico, F., Stefanutti, C., Fazio, C., Cantini, R., Ricci, G., Trichopoulou, A., and Katapoti, J. (1986). A multi-centre study on completeness of urine collection in 11 European centres. I. Some problems with the use of creatinine and 4-aminobenzoic acid as marker of the completeness of collection. Human Nutr. Clin. Nutr. 40, 229-237. Lee, E. W., Terzo, T. S., D'Arcy, J. B., Gross, K. B., and Schreck, R. M. (1992). Lack of blood formate accumulation in humans following exposure to methanol vapor at the current permissible exposure limit of 200 ppm. Am. Ind. Hyg. Assoc. J. 53, 99-104.[Medline] Liesivuori, J., and Savolainen, H. (1991). Methanol and formic acid toxicity: Biochemical mechanisms. Pharmacol. Toxicol. 69, 157-163.[Medline] McMartin, K. E., Martin-Amat, G., Makar, A. B., and Tephly, T. R. (1977). Methanol poisoning. V. Role of formate metabolism in the monkey. J. Pharmacol. Exp. Ther. 201, 564-572.[Medline] McMartin, K. E., Martin-Amat, G., Noker, P. E., and Tephly, T. R. (1979). Lack of role for formaldehyde in methanol poisoning in the monkey. Biochem. Pharmacol. 28, 645-649.[Medline] Medinsky, M. A., Dorman, D. C., Bond, J. A., Moss, O. R., Janszen, D. B., and Everitt, J. I. (1997). Pharmacokinetics of methanol and formate in female cynomolgus monkeys exposed to methanol vapors. Res. Rep. Health Eff. Inst. 77,1-30; discussion 31-38.[Medline] Morikawa, S., and Harada, T. (1969). Immunohistochemical localization of catalase in mammalian tissues. J. Histochem. Cytochem. 17, 30-5.[Medline] Nelson, B. K., Brightwell, S., MacKenzie, D. R., Khan, A., Burg, J. R., Weigel, W. W., and Goad, P. T. (1985). Teratological assessment of methanol and ethanol at high inhalation levels in rats. Fundam. Appl. Toxicol. 5, 727-736.[Medline] Nihlén, A., and Droz, P.-O. (2000). Toxicokinetic modelling of methyl formate exposure and implications for biological monitoring. Int. Arch. Occup. Environ. Health 73, 479-487.[Medline] Osterloh, J. D., D'Alessandro, A., Chuwers, P., Mogadeddi, H., and Kelly, T. J. (1996). Serum concentrations of methanol after inhalation at 200 ppm. J. Occup. Environ. Med. 38, 571-576.[Medline] Palese, M., and Tephly, T. R. (1975). Metabolism of formate in the rat. J. Toxicol. Environ. Health 1, 13-24.[Medline] Perkins, R. A., Ward, K. W., and Pollack, G. M. (1995). A pharmacokinetic model of inhaled methanol in humans and comparison to methanol disposition in mice and rats. Environ. Health Perspect. 103, 726-733.[Medline] Perkins, R. A., Ward, K. W., and Pollack, G. M. (1996). Methanol inhalation: Site and other factors influencing absorption, and an inhalation toxicokinetic model for the rat. Pharm. Res. 13, 749-55.[Medline] Pollack, G. M., and Brouwer, K. L. (1996). Maternal-fetal pharmacokinetics of methanol. Res. Rep. Health Eff. Inst. 74,1-48; discussion 49-53.[Medline] Pollack, G. M., Brouwer, K. L., and Kawagoe, J. L. (1993). Toxicokinetics of intravenous methanol in the female rat. Fundam. Appl. Toxicol. 21, 105-110.[Medline] Røe, O. (1982). Species differences in methanol poisonings. Crit. Rev. Toxicol. 10, 275-286.[Medline] Sedivec, V., Mraz, M., and Flek, J. (1981). Biological monitoring of persons exposed to methanol vapours. Int. Arch. Occup. Environ. Health 48, 257-271.[Medline] Sejersted, O. M., Jacobsen, D., Ovrebo, S., and Jansen, H. (1983). Formate concentrations in plasma from patients poisoned with methanol. Acta Med. Scand. 213, 105-110.[Medline] Sugata, Y., Halbach, S., Allen, J., and Clarkson, T. W. (1979). Tissue distribution of inhaled mercury vapor in acatalasemic mice. J. Environ. Sci. Health C 13, 97-106.[Medline] Tephly, T. R., and McMartin, K. E. (1984). Methanol metabolism and toxicity. In Aspartame. Physiology and Biochemistry (L. D. Stegink and L. J. Filer, Jr., Eds.), pp. 111-140. Marcel Dekker, New York. U.S. DHHS (1993). Methanol toxicity. Agency for Toxic Substances and Disease Registry. Am. Fam. Physician 47, 163-171.[Medline] Ward, K. W., Blumenthal, G. M., Welsch, F., and Pollack, G. M. (1997). Development of a physiologically based pharmacokinetic model to describe the disposition of methanol in pregnant rats and mice. Toxicol. Appl. Pharmacol. 145, 311-322.[Medline] Ward, K. W., Perkins, R. A., Kawagoe, J. L., and Pollack, G. M. (1995). Comparative toxicokinetics of methanol in the female mouse and rat. Fundam. Appl. Toxicol. 26, 258-264.[Medline] Ward, K. W., and Pollack, G. M. (1996). Comparative toxicokinetics of methanol in pregnant and nonpregnant rodents. Drug. Metab. Dispos. 24, 1062-1070.[Abstract] Yasugi, T., Kawai, T., Mizunuma, K., Horiguchi, S., Iwami, O., Iguchi, H., and Ikeda, M. (1992). Formic acid excretion in comparison with methanol excretion in urine of workers occupationally exposed to methanol. Int. Arch. Occup. Environ. Health 64, 329-37.[Medline] ************************************************************** Gosselin NH, Brunet RC, Carrier G. Comparative occupational exposures to formaldehyde released from inhaled wood product dusts versus that in vapor form. Appl Occup Environ Hyg. 2003 May; 18(5): 384-93. PMID: 12746082 Bouchard M, Gosselin NH, Brunet RC, Samuel O, Dumoulin MJ, Carrier G. Michèle Bouchard, Nathalie H. Gosselin, Robert C. Brunet, Onil Samuel, Marie-Josée Dumoulin, and Gaétan Carrier A toxicokinetic model of malathion and its metabolites as a tool to assess human exposure and risk through measurements of urinary biomarkers. Toxicol Sci. 2003 May; 73(1): 182-94. Epub 2003 Mar 25. Erratum in: Toxicol Sci. 2003 Aug; 74(2): following table of contents. PMID: 12657741 Carrier G, Bouchard M, Brunet RC, Caza M. A toxicokinetic model for predicting the tissue distribution and elimination of organic and inorganic mercury following exposure to methyl mercury in animals and humans. II. Application and validation of the model in humans. Toxicol Appl Pharmacol. 2001 Feb 15;171(1):50-60. PMID: 11181111 Carrier G, Brunet RC, Caza M, Bouchard M. A toxicokinetic model for predicting the tissue distribution and elimination of organic and inorganic mercury following exposure to methyl mercury in animals and humans. I. Development and validation of the model using experimental data in rats. Toxicol Appl Pharmacol. 2001 Feb 15;171(1):38-49. PMID: 11181110 ************************************************************** http://groups.yahoo.com/group/aspartameNM/message/1100 research on aspartame (methanol, formaldehyde, formic acid) toxicity: Murray 2004.08.02 rmforall [ 2004.11.16 additions ] Rich Murray, MA Room For All rmforall@comcast.net 1943 Otowi Road, Santa Fe, New Mexico 87505 USA 505-501-2298 http://groups.yahoo.com/group/aspartameNM/messages 137 members, 1,142 posts in a public searchable archive The moderated newsgroup, bionet.toxicology , has accepted 25 of my long reviews since March 24: Dr. Charles "Chuck" A. Miller III rellim@tulane.edu Associate Professor of Environmental Health Sciences 374 Johnston Building, SL29 Tulane Univ. School of Public Health and Tropical Medicine 1430 Tulane Avenue New Orleans, LA 70112 (504)585-6942 Bionet.toxicology news group http://www.bio.net/hypermail/toxicol/current [ NutraSweet, Equal, Canderel, Benevia, E951 ] http://groups.yahoo.com/group/aspartameNM/message/927 Donald Rumsfeld, 1977 head of Searle Corp., got aspartame FDA approval: Turner: Murray 2002.12.23 rmforall A very detailed, highly credible account of the dubious approval process for aspartame in July, 1981 is part of the just released two-hour documentary "Sweet Misery, A Poisoned World: An Industry Case Study of a Food Supply In Crisis" by Cori Brackett: cori@soundandfuryproductions.com http://www.soundandfuryproductions.com/ 520-624-9710 2301 East Broadway, Suite 111 Tucson, AZ 85719 http://groups.yahoo.com/group/aspartame/messages Aspartame Victims Support Group Edward Bryant Holman, Chief Moderator 826 members, 17,766 posts in a public, searchable archive http://www.presidiotex.com/aspartame/ bryanth@presidiotex.net http://www.HolisticMed.com/aspartame mgold@holisticmed.com Aspartame Toxicity Information Center Mark D. Gold also Co-Moderator 12 East Side Drive #2-18 Concord, NH 03301 603-225-2110 http://www.holisticmed.com/aspartame/abuse/methanol.html "Scientific Abuse in Aspartame Research" http://groups.yahoo.com/group/aspartameNM/message/1131 genotoxicity of aspartame in human lymphocytes 2004.07.29 full plain text, Rencuzogullari E et al, Cukurova University, Adana, Turkey 2004 Aug: Murray 2004.11.06 rmforall "Schwartz ( 1999 ) also reported that methanol is converted to formaldehyde which then accumulates in the cells. Formaldehyde has been considered an inducer of cancer and acts to alter DNA ( Ewertz, 1993; Ewertz and Gill, 1990 ). Olney et al. ( 1996 ) reviewed and explained that ASP had mutagenic potential..... In this study, we found that, ASP did appear to have genotoxic potential consistent with potential carcinogenicity. According to these results, phenyalanine and methanol, which are metabolic products of ASP, have a genotoxic risk for humans. In contrast, ASP was not found as a mutagen in in vivo studies. However, in the present study, ASP induced CA and micronuclei in human lyphocytes dose-dependently. ASP did not change the osmolality of the medium at the maximum concentrations ( 346 milliosmol) when compared with untreated medium (342 milliosmol ). It was reported that a deviation from physiological osmolality ( approximately 300 milliosmol ) can lead to genotoxic effects ( Nowak, 1984, 1997; Seeberg et al., 1989 ). According to these results, we can conclude that ASP induced CA and percentage of micronuclei by itself because it did not alter the pH and osmolality of the medium. As shown, there are several contradictory studies about genotoxicity and carcinogenicity of ASP. However, it must be taken into account that ASP induced the CA and micronuclei formation in a dose-dependent manner. It is not possible to conclude that ASP is safe according to these results. Therefore, it is necessary to be careful when using it in food and beverages as a sweetener." Genotoxicity of aspartame 2004.07.29 plain text, Rencuzogullari E et al, Cukurova University, Adana, Turkey 2004 Aug Drug Chem Toxicol. 2004 Aug; 27(3): 257-68. Genotoxicity of aspartame. reyyup@mail.cu.edu.tr Rencuzogullari E, Tuylu BA, Topaktas M, Ila HB, Kayraldiz A, Arslan M, Diler SB. Biology Department, Faculty of Arts and Sciences, Natural and Applied Sciences Institute, Cukurova University, Adana, Turkey. Poor memory is one of the main early complaints of aspartame reactors, who are often people who use over 6 cans ( 2 L) diet soda daily for years. The 12 experimental rats in this recent economical, focused study by McConnaughey M et al (2004 May), drank a comparable level for 4 months, about 13% of a 30-month lifespan. It is an excellent introduction to the main issues. Only after 3 months did the 12 aspartame rats show almost a doubling of time to run a single-choice maze. At 4 months, there was almost another doubling of delay: "...two of the treated rats even went to the wrong side of the T-maze, totally forgetting where the reward was." These are very powerful, worrisome results. There were highly significant, neurologically relevant changes in certain brain receptor densities, and changes in brain chemistry. With 70 citations, the relevant scientific literature is well summarized. Many other studies, often industry funded, often used single doses or too short durations of exposure, along with lower doses, thus rarely proving memory deficits. The funding source for this extremely valuable study is not given. It used a team of talented high school students. The fact that certain brain receptor densitities increased, and that memory deficit increase took 3 months to be significant, may reflect the paradox of hormesis, the complex ability of organisms to make themselves stronger in response to low levels of toxins: http://groups.yahoo.com/group/aspartameNM/message/1055 hormesis: possible benefits of low-level aspartame (methanol, formaldehyde) use: Calabrese: Soffritti: Murray 2004.03.11 rmforall The most toxic part of the fragile aspartame molecule is its 11% methanol component. It is an open secret, admitted in a number of published studies for three decades, that methanol is converted within hours by the liver into formaldehyde and formic acid, both potent, cumulative toxins that affect all cell types. Few know that the classic "morning after" hangover from dark wines and liquors is largely due to formaldehyde and formic acid from methanol contamination, not the ethanol itself. The actual disposition of these toxins in the tissues of human aspartame reactors has never been determined, or, if determined, never publicly published. The study should be replicated, using methanol, formaldehyde, and formic acid to verify if the same results obtain. If blood and tissue samples have been stored, then the fast, cheap, automated, highly sensitive Comet assay, often used to prove DNA damage from formaldehyde, can be used to replicate the results by Yu F. Sakaki (2002), whose intripid and much published team in Japan has found DNA damage, testing 8 tissues from single non-lethal doses of aspartame (near-significant high levels of DNA damage in 5 tissues) and 38 other additives in groups of just 4 mice: http://groups.yahoo.com/group/aspartameNM/message/935 Comet assay finds DNA damage from sucralose, cyclamate, saccharin in mice: Sasaki YF & Tsuda S Aug 2002: Murray 2003.01.01 rmforall [ Also borderline evidence, in this pilot study of 39 food additives, using test groups of 4 mice, for DNA damage from for stomach, colon, liver, bladder, and lung 3 hr after oral dose of 2000 mg/kg aspartame-- a very high dose. Methanol is the only component of aspartame that can lead to DNA damage. ] http://groups.yahoo.com/group/aspartameNM/message/1088 Murray, full plain text & critique: chronic aspartame in rats affects memory, brain cholinergic receptors, and brain chemistry, Christian B, McConnaughey M et al, 2004 May: 2004.06.05 rmforall Pharmacol Biochem Behav. 2004 May; 78(1): 121-7. Chronic aspartame affects T-maze performance, brain cholinergic receptors and Na(+),K(+)-ATPase in rats. Christian B, McConnaughey K, Bethea E, Brantley S, Coffey A, Hammond L, Harrell S, Metcalf K, Muehlenbein D, Spruill W, Brinson L, McConnaughey M. Department of Pharmacology, Brody School of Medicine, East Carolina University, Greenville, NC 27858, USA; North Carolina School of Science and Mathematics, Durham, NC 27811. http://www.ecu.edu/pharmacology/faculty/mcconnaughey.html Mona M. McConnaughey, Ph.D. Research Assistant Professor Department: PHARMACOLOGY & TOXICOLOGY Office: Brody Medical Science 6E-120A 252-744-2756 MCCONNAUGHEYM@mail.ecu.edu This study demonstrated that chronic aspartame consumption in rats can lead to altered T-maze performance and increased muscarinic cholinergic receptor densities in certain brain regions. Control and treated rats were trained in a T-maze to a particular side and then periodically tested to see how well they retained the learned response. Rats that had received aspartame (250 mg/kg/day) in the drinking water for 3 or 4 months showed a significant increase in time to reach the reward in the T-maze, suggesting a possible effect on memory due to the artificial sweetener. Using [(3)H]quinuclidinyl benzilate (QNB) (1 nM) to label muscarinic cholinergic receptors and atropine (10(-6) M) to determine nonspecific binding in whole-brain preparations, [ the 12 ] aspartame-treated rats showed a 31% increase in receptor numbers when compared to controls. In aspartame-treated rats, there was a significant increase in muscarinic receptor densities in the frontal cortex, midcortex, posterior cortex, hippocampus, hypothalamus and cerebellum of 80%, 60%, 61%, 65%, 66% and 60%, respectively. The midbrain was the only area where preparations from aspartame-treated rats showed a significant increase in Na(+),K(+)-ATPase activity. It can be concluded from these data that long-term consumption of aspartame can affect T-maze performance in rats and alter receptor densities or enzymes in brain. PMID: 15159141 http://groups.yahoo.com/group/aspartameNM/message/1067 eyelid contact dermatitis by formaldehyde from aspartame, AM Hill & DV Belsito, Nov 2003: Murray 2004.03.30 rmforall [ 150 KB ] "A 60-year-old Caucasian woman presented with a 6-month history of eyelid dermatitis... By strictly avoiding formaldehyde and all formaldehyde releasers for the next 3 weeks, she improved only slightly. Her problem, however, was subsequently solved when a local pharmacist advised her to avoid aspartame. She had begun using an aspartame-based artificial sweetener 5 months prior to the onset of her dermatitis. [ 12 months of low-level aspartame use until stopping. ] Within 1 week of discontinuing the aspartame, her eyelid dermatitis resolved completely and has not recurred over 18 months without specific treatment.... Our patient was consuming an average of 80 mg (1.13 mg/kg) of aspartame daily, well below the levels previously studied." [ A packet of tabletop sweetener gives 37 mg aspartame, while a 12 oz diet soda gives 200 mg aspartame. An aspartame reactor can have immediate strong symtoms from an under-the-tongue wafer with 4 mg aspartame. ] http://groups.yahoo.com/group/aspartameNM/message/1039 three-page review: aspartame (methanol, formaldehyde) toxicity: Murray 2003.11.22 rmforall http://groups.yahoo.com/group/aspartameNM/message/1026 brief aspartame review: formaldehyde toxicity: Murray 2003.09.11 rmforall http://groups.yahoo.com/group/aspartameNM/message/1025 aspartame & formaldehyde toxicity: Murray 2003.09.09 rmforall http://groups.yahoo.com/group/aspartameNM/message/1094 the 11% methanol component of aspartame becomes formaldehyde, now ruled a carcinogen by WHO International Agency for Research on Cancer: Murray 2004.06.16 rmforall http://groups.yahoo.com/group/aspartameNM/message/1084 26 stevia safety abstracts since 1993: aspartame vs stevia debate on alt.support.diabetes, George Schmidt, OD: Murray 2004.05.25 rmforall http://groups.yahoo.com/group/aspartameNM/message/1133 Mark Gold, most recent of 14 Rapid Responses to Aspartame and its effects on health, BMJ: Murray 2004.11.06 rmforall http://groups.yahoo.com/group/aspartameNM/message/1124 8 more Rapid Responses to Aspartame and its effects on health, BMJ: Murray 2004.10.18 rmforall http://groups.yahoo.com/group/aspartameNM/message/1120 5 critical Rapid Responses to Aspartame and its effects on health, Michael E J Lean and Catherine R Hankey, BMJ 2004; 329: 755-756: Murray 2004.10.05 rmforall http://groups.yahoo.com/group/aspartameNM/message/1117 Aspartame and its effects on health, Michael E.J. Lean, Catherine R. Hankey, Glasgow UK, British Medical Journal: 11% methanol component of aspartame, and same level of methanol in dark wines and liquors, turns to formaldehyde and formic acid, the main cause of chronic hangover symptoms: Murray 2004.10.04 rmforall http://bmj.bmjjournals.com/cgi/eletters/329/7469/755#76712 *************************************************************** groups.yahoo.com/group/aspartameNM/messages
Report this post as:
|